
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
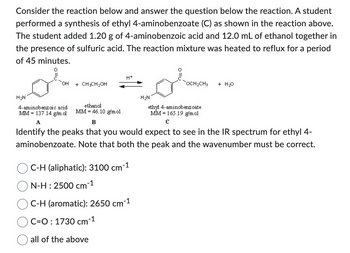
Transcribed Image Text:Consider the reaction below and answer the question below the reaction. A student
performed a synthesis of ethyl 4-aminobenzoate (C) as shown in the reaction above.
The student added 1.20 g of 4-aminobenzoic acid and 12.0 mL of ethanol together in
the presence of sulfuric acid. The reaction mixture was heated to reflux for a period
of 45 minutes.
H₂N
OH
4-aminobenzoic acid
MM 137.14 g/m ol
A
+ CH3CH₂OH
ethanol
MM 46.10 g/mol
H+
C-H (aliphatic): 3100 cm¯
-1
N-H: 2500 cm¯1
or
ethyl 4-aminobenzoate
MM 165.19 g/mol
с
C-H (aromatic): 2650 cm-¹
C=O: 1730 cm-1
all of the above
H₂N
B
Identify the peaks that you would expect to see in the IR spectrum for ethyl 4-
aminobenzoate. Note that both the peak and the wavenumber must be correct.
OCH₂CH3 + H₂O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Indicate whether the following reactions and phase changes are exothermic or endothermic: a)O2(g) →O2(l) b)2 CH3OH + 3 O2→2 CO2+ 4 H2O. ΔH = -23 cal c)2 H2O + 45 cal →O2+ 2 Harrow_forwardEnter a balanced equation for the complete combustion of liquid C3H6OC3H6O. Express your answer as a chemical equation. Identify all of the phases in your answer.arrow_forwardThis problem has been solved! See the answer For the reactionH2 (g) + Br2 (g) ↔ 2 HBr (g),K = 4.0 x 10-2. For the reaction2 HBr (g) ↔ H2 (g) + Br2 (g)K =:a. 4.0 x 10-2b. 5c. 25d. 2.0 x 10-1 why is it 25?arrow_forward
- 9 Complete the following reactions by briefly explaining in each case the type of reaction taking place. ii. H₂ Pd/C ??? ??? + oro.o OH + OHarrow_forwardConsider The Following Reaction: CaCO3(S) + 2HCl(Aq) = CaCl2(Aq) + H2O(L) + CO2(G) Write down the quation of the equilibrium constant kc for this reactionarrow_forwardCaluclate change H for the reaction 4 NH3 (g) + 5 O2 (g) -> 4 NO (g) + 6 H2O (g).arrow_forward
- Please solve both A and B. Thanks!arrow_forwardFor each of the following reactions, choose which expression represents Ke. a. TIC13 (8) TICI(s) + Cl₂ (9) [TIC13] [TICI] [C1₂] O Ke OKc = [Cl₂] [TICI] [C1₂] [TIC13] 2- b. CuCl₂² (aq) → Cu²+ (aq) + 4Cl¯ (aq) Ke OKC Ke = Кс Oke Oke = O Kc = = = c. 302 (g) 203 (9) [0₂]³ [03]² [03]² = = [Cu²+ ][CI-14 [CuCl2] [CuCl2-] = [Cu²+ ][CI] [Cu²+ ][CI] [CuCl2] [02] ³ [03] ³ 10.1²arrow_forward1) Use Hess's Law to determine AH for the following target reaction. 3 CO₂(g) + 4 H₂O(g) b) C3Hg(g) + 5 O₂(g) C(s) + O₂(g) -CO₂(g) 2 H₂(g) + O₂(g) 3 C(s) + 4 H₂(g) →→→ 2 H₂O(g) C3H8(g) AH = AH = ΔΗ ΔΗ = ??? -2043 kJ -393.5 kJ -483.6 kJarrow_forward
- 3. Answer all sections (i) - (iv). 1 mole of ethanol (C2H5OH) and 1 mole of ethanoic acid (CH;COOH) were reacted at 25 °C, in a non- aqueous solvent, until equilibrium was reached. It was subsequently determined that 0.33 moles of ethanol remained at equilibrium. (i) Write an equation for the reaction. (ii) Write an expression for the equilibrium constant, Kc, for the reaction and calculate its value. Subsequently a further 3 moles of C2H5OH was added to the solution. (ii) Determine the concentrations of all the species present when equilibrium is restored.arrow_forwardplease answer fast! Cyclohexane (C6H12) undergoes a molecular rearrangement in the presence of AIC13 to form methylcyclopentane (MCP) according to the equation: C_{6}*H_{12} = MCP If K_{C} = 0.143 at 25°C for this reaction, predict the direction in which the system will shift if the initial concentrations of C6H12 and MCP are 0.200 M and 0.100 M, respectively. The system A) will shift right B) will shift left. C) is already at equilibrium. D) is not at equilibrium and will remain in an unequilibrated state.arrow_forwardSee image below. Can you please show me how to solve this problem?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY