
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
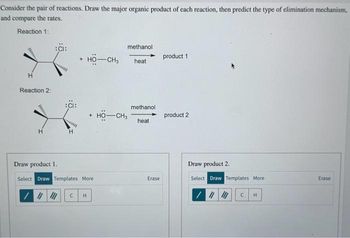
Transcribed Image Text:Consider the pair of reactions. Draw the major organic product of each reaction, then predict the type of elimination mechanism,
and compare the rates.
Reaction 1:
www
H
Reaction 2:
:CI:
:CI:
HO-CH3
+ HỘ—CH,
Draw product 1.
Select Draw Templates More
/ || ||| C H
methanol.
heat
methanol
heat
Erase
product 1
product 2
Draw product 2.
Select Draw Templates More
/
||||||
H
Erase

Transcribed Image Text:Identify the mechanism of each reaction.
Reaction 2 occurs by an E2 mechanism.
Reaction 1 occurs by an E2 mechanism.
Reaction 2 occurs by an El mechanism.
Reaction 1 occurs by an El mechanism.
Compare the rates of each reaction.
Reaction 1 is faster than reaction 2.
Both reactions occur at the same rate.
Reaction 2 is faster than reaction 1.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- please do last 4arrow_forwardMechanism: A reaction mechanism for the following reaction is shown below. H+ CEN CEN: N-H || -C-OH H₂O, H* Step 1 wand woled mot ozsm E Step 3 N-H C-OH C=N-H Step2 C=N-H H-O-H H₂O motno vgiene fesrigid onlt to smotnos -C=N-H a) The overall reaction is an example of b) Step 1 is c) Step 3 is d) Draw in the curved arrows for each step. e) Identify the nucleophiles and electrophiles where appropriate. f) Fill in the reaction energy diagram. H₂O: rate determining step to noipojovo hamwell sit 5(emise erit voittons 10) vanas mi sdgin al rainW di of E^ reaction progress →arrow_forwardChemistry please answer in tipping formatarrow_forward
- Give detailed answer with explanation neededarrow_forwardMechanism: A reaction mechanism for the following reaction is shown below. H+ CEN CEN: N-H || -C-OH H₂O, H* Step 1 Step 3 N-H C-OH C=N-H H₂O Step2 .C=N-H H-O-H Semse anls a) The overall reaction is an example of b) Step 1 is c) Step 3 is d) Draw in the curved arrows for each step. e) Identify the nucleophiles and electrophiles where appropriate. f) Fill in the reaction energy diagram. C=N-H H₂O: rate determining step E个 reaction progress →arrow_forwardWhich alkene is major product of the E2 reaction below? Options: 1 2 3 4arrow_forward
- all one question, draw products and chose what reagent it isarrow_forwardBoth dehydration reactions go through carbocation rearrangements. Does the mechanism in each reaction involve a hydride shift or a methyl shift? O Hold and drag to reorder 1. = Hydride shift = Hydride shift = Methyl shift = Methyl shift H,SO, 1) heat OH OH H,SO, 2) heat 2.arrow_forwardDraw the major organic substitution product for the reaction shown. Select Draw Rings More Erase H CI CH;CH2OH isopropyl alcoholarrow_forward
- Draw the major organic product when each compound is treated with Tollens' reagent. If no reaction occurs, draw the reactant. Reaction A Select Draw Rings More Erase / |||||| C 0 H Ag* uz ü H OH™ HO S Ć Q2 Q Reaction B Select Draw Erase / // / C HO Ag+ OH- O Rings H More OH₂arrow_forwardDraw the alkyne formed when 1,1-dichloro-3-methylbutane is treated with an excess of a strong base such as sodium amide. Omit byproducts. (Give the neutralized alkyne product, not the acetylide salt.) 9 excess NaNH, Draw the neutral product. Select Draw / || ||| C Rings More H Cl Erasearrow_forward19) Identify (circle) all possible products formed by the reaction depicted below CI wwwwww... MeOH heat OMe MeOarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY