
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
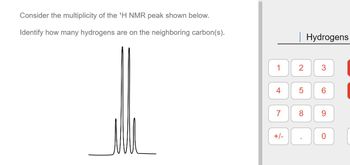
Transcribed Image Text:**Consider the multiplicity of the ¹H NMR peak shown below.**
**Identify how many hydrogens are on the neighboring carbon(s).**
### Explanation of the NMR Peak:
The image shows an NMR spectrum with a peak that appears as a quintet, indicating five distinct splitting patterns. This suggests that there are four hydrogen atoms on the neighboring carbon atoms. According to the N+1 rule in NMR spectroscopy, the number of peaks (multiplicity) is equal to the number of neighboring hydrogens plus one. Thus, a quintet corresponds to four neighboring hydrogens.
### Hydrogen Selection Panel:
On the right side of the image, there is a numerical keypad with numbers from 1 to 9, a plus/minus button, a decimal point, and a zero. This allows the user to input the number of hydrogens on neighboring carbon(s), based on their analysis of the NMR peak’s multiplicity.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Match each of the following compounds to one of the HNMR spectra shown below.arrow_forwardThe simulataed APT spectrum of a compound with the molecular formula C8H16 is shown. Draw a structure that is consistent with this data.arrow_forward14. How many distinct signals are in the ¹³C NMR of the following compound?arrow_forward
- 2. Draw structures for compounds that have the proton nmrs described: a) C2H6O; one singlet b) CAH&Cl2O; two triplets c) CAHBO2; one singlet, one triplet and one quartetarrow_forwardIn the attached H1-NMR, draw and identify each set of equivalent hydrogens in the structure and list peak they belong to in the NMR spectrum.arrow_forward1c) What are the expected NMR and IR for the following compound? Q₂N-arrow_forward
- The protons indicated in the structure below would show up as a spectrum. O singlet O doublet O triplet O quartet Oseptet CH3 CH3 in a proton NMRarrow_forwardSelect the compound from each group that matches the HNMR spectrum shown below.arrow_forwardIdentify the following spectra. Assign all pertinent peaks (~5-6 peaks). Place the structure of the compound in the box on the lower left-hand corner of spectrum. Please give explanation and draw on diagram as well, thank youarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY