
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN: 9781938168390
Author: Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
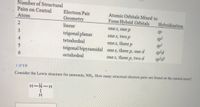
Transcribed Image Text:Number of Structural
Pairs on Central
Electron Pair
Atomic Orbitals Mixed to
Atom
Geometry
Form Hybrid Orbitals
Hybridization
linear
one s, one p
sp
trigonal planar
one s, two p
sp
sp³
sp'd
sp'd?
4
tetrahedral
one s, three p
trigonal bipyramidal ones, three p, one d
6.
octahedral
one s, three p, two d
1 of 9
Consider the Lewis structure for ammonia, NH3. How many structural electron pairs are found on the central atom?
H-N-H
H.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify all the central atoms that are sp°d2-hybridized in the following molecule: 3- : Br- Kr- PO23- XeF2 KrBrCl2F * SO2 PCI5 CIF;S :5:arrow_forwardWhat are the respective hybridizations of the atoms numbered 1 to 4 in this compound? 0: N-CH2-C -NH-CH-CEN: 1 2 4 O sp2;sp3;sp2;sp O sp2;sp2;sp3;sp O sp3;sp2;sp2;sp2 O sp3;sp2;sp3;sparrow_forward(b )Predict the bond angle indicated with (?) between the lone pair and the equatorial F in SF4? 28. (20 *IA S):exie a) 120° ni o 1ebro ni tall gaiwollot a b) 901amagnsvs 1ec A (ainiog ).81 S F C)greater than 120° d) less than 120° $2>0 > 6M A (S F : c) than 120° (darrow_forward
- 1. Molecule A, shown below is closely related to the drug NGD-4715, which exhibits anti- depressant effects in animal models. Which of the following best describes the orbital overlap between the C and N atoms that provides the G-bond in the cyano group (labelled "e")? (A) C sp - N sp (D) Cp-Np OA OB OC OD OH (B) C sp'-N p (E) C sp - N sp¹ Molecule A (C) C sp - N sparrow_forwarddetermine the molecular structure based on the spectroscopic data in the picture explain for each picturearrow_forwardThe molecular orbital diagram of NO shown in Figure 10.47 also applies to the following species. Write the molecular orbital electron configuration of each, indicating the bond order and the number of unpaired electrons. (a) LiBe+ (b) CO+ (c) CN (d) OF Figure 10.47 Molecular orbital diagram for nitric oxide (NO). The molecular orbital diagram for NO predicts a bond order of 2.5 and predicts that the molecule is paramagnetic with one unpaired electron. These predictions are verified by experimental measurements.arrow_forward
- Draw an orbital picture of thiazole. Assume that both the nitrogen and sulfur atoms are sp2-hybridized, and show the orbitals that the lone pairs occupy.arrow_forwardConsider any one of the four identical hybrid orbitals in the 109.5° set. a. What fraction of the clay in this hybrid orbital was originally red (carne from the 2s orbital)? b. What fraction of the clay in this hybrid orbital was originally green (carne from a 2porbital)? c. Explain the name “ s(1/4)p(3/4) hybrid orbital” for each of the four orbitals. d. In fact, each of the four hybrid orbitals in the 109.5° set is called an sp3 -hybrid orbital.Explain the name “ sp3 -hybrid orbital.”arrow_forwardWhich compound in each of the following pairs would you expect to have the greater dipole moment u? Why? (a) HF or HCI (b) HF or BF3 (c) (CH3); CH or (CH3)3CCI (d) CHCI3 or CCl;F (e) CH3 NH2 or CH3 OH (f) CH3 NH2 or CH3 NO2arrow_forward
- Excel File Edit View Insert Format Tools Data Window Help AutoSave 72% O Sun 8:33 PM O. a thermo-Last Modified: 29m ago Home Insert Draw Page Layout Formulas Data Review View Tell me 2 Share Calibri (Body) v 12 v A A ab ab General Insert v Σ Paste BIUV 田、 Delete v 州网 即加 $ v % 9 、白-白 .00 Conditional Format Cell Sort & Filter Find & Select Analyze Data Formatting as Table Styles Format v Sensitivity fx 1/T A B. E 1/T In Ksp K P R 5 T. 0.00353 -4.85 0.00328 -3.36 0.00308 -1.18 titration 0.0029 0.93 0.0005 0.001 0.0015 0.002 0.0025 0.003 0.0035 0.004 y=-9231.1x + 27.402 R = 0.9762 1/T Sheet1 Count: 10 Sum: -8.44721 + 10 Average: -1.05590125 灵國 TO tv W 2. 海 டாடarrow_forwardOn one-electron oxidation of a five valence electron AH₂ compound (C₂, point group) to a closed shell (no unpaired electrons) four valence electron C₂, cationic species, what is the symmetry of the highest energy occupied molecular orbital, and what happens to the energy of this orbital when the four valence electron AH2 C2y cation changes to a Doch geometry? O 2a₁ and stabilised (decreases in energy) O 2a₁ and destabilised (increases in energy) O 1b2 and stabilised (decreases in energy) O b₁ and no change in energyarrow_forwardPlease don't provide handwritten solution .....arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
