
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
1.
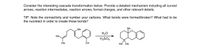
Transcribed Image Text:**Title: Understanding Cascade Transformations in Organic Chemistry**
**Description:**
Consider the interesting cascade transformation below. Provide a detailed mechanism including all curved arrows, reaction intermediates, reaction arrows, formal charges, and other relevant details.
**TIP:** Note the connectivity and number your carbons. What bonds were formed/broken? What had to be the nucleophile in order to create those bonds?
**Reaction Overview:**
In the initial structure, a complex organic compound contains three methyl groups (Me), two of which are attached to a single carbon. A double bond and an alcohol group (OH) are present in the linear chain adjacent to the three-carbon ring.
- **Reactants:**
- The substrate with a hydroxyl group (OH) and multiple methyl groups (Me).
- Reagents: water (H₂O) and sulfuric acid (H₂SO₄) are used to facilitate the reaction.
- **Product:**
- The resultant product shows a new cyclic structure where the previous alcohol group is transformed, suggesting the formation of a ketone group (indicated by O at the position previously OH). The original linear chain has undergone cyclization.
This transformation implies a sequence of reactions where specific new bonds are formed while others are broken, mediated by acid-catalyzed dehydration, rearrangements, or cyclization processes.
Understanding this transformation involves detailing the mechanism, identifying nucleophilic attacks, electrophile positions, and observing how the structural connectivity changes from reactants to product.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1.15 Add coefficients to balance the chemical equations shown. a. Al(s) + Cl₂(g) → AlCl3(s) b. HCl(aq) + Zn(s) → ZnCl₂(aq) + H₂(g) S INGINE c. SiO₂ (s) + C(s) → SiC(s) + CO(g)arrow_forwardQ13.arrow_forward< ... F6 The U.S. quarter has a mass of 5.67 g and a thickness of 1.50 mm. How may pounds would a stack of quarters weigh if you stacked them as high as the Eiffel Tower? The Eiffel tower has a height of 986 feet. Remember: 1 pound = 454 g 1 cm = 10 mm 1 inch = 2.54 cm 1 foot 12 inches F7 1 4 7 +/- Question 12 of 12 DII F8 2 5 8 . DD F9 pounds 3 6 9 O F10 C x 100 F11 Submi F12arrow_forward
- 4. Make the following conversions and include an equation for each one:(a) 32 ℃ to ℉ (c) 273 ℃ to K(b) -8.6 ℉ to ℃ (d) 100 K to ℉arrow_forward7. 12.0 mL of A weighs 10.8 g; and 16.0 mL of B weighs 14.4 g. A. A has a higher density than B. B. B has a higher density than A. C. A and B have equal densities. D. There is insufficient information to answer this question.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY