
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
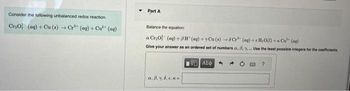
Transcribed Image Text:Consider the following unbalanced redox reaction.
Cr₂O² (aq) + Cu(s) → Cr³+ (aq) + Cu²+ (aq)
Part A
Balance the equation
a Cr₂0 (aq) +8H (aq) + Cu(s) +6C+ (aq)+cH₂O(l) + Cu²+ (aq)
Give your answer as an ordered set of numbers a. 8. y. Use the least possible integers for the coefficients.
ΑΘΕ ΑΣΦΑ
a. B. 7.6.c.k
→
?
![Part B
Determine the volume of a 0.800 mol L-¹ K₂Cr₂O7 solution required to completely react with 5.20 g of Cu.
V =
[5] ΑΣΦ
20 PAR ?
mL](https://content.bartleby.com/qna-images/question/93650a4e-193c-4d0e-a1cd-f2051a53af97/6d84ac3e-0c7a-4c88-9512-42590bd2c084/00l68h_thumbnail.jpeg)
Transcribed Image Text:Part B
Determine the volume of a 0.800 mol L-¹ K₂Cr₂O7 solution required to completely react with 5.20 g of Cu.
V =
[5] ΑΣΦ
20 PAR ?
mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write balanced half-reactions for the following redox reaction: 8 CO₂(aq) + AsH3(g)+8 OH¯(aq) → 4C₂0¾¯(aq) + H₂AsO 4(aq) + 4H₂O(1) reduction: 0 oxidation: ロ→ロ e X Sarrow_forwardBalance the following redox equation, for a reaction which takes place in basic solution. HS (aq) + ClO3(aq) → S(s) + Cl(aq) (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) - HS (aq) + ClO3(aq) + S(s) Cl(aq) +arrow_forwardUse the relative strengths of nonmetals and metals as oxidizing and reducing agents, as indicated in the following unbalanced equations, to construct a table of half‐reactions. Ag (s) + Br2 (l) → AgBr (s) Ag (s) + I2 (s) → no evidence of reaction Cu2+ (aq) + I‐ (aq) → no redox reaction Br2 (l) + Cl‐ (aq) → no evidence of reactionarrow_forward
- Complete and balance the following redox equations using the smallest whole-number coefficients. The reaction takes place in a BASIC solution. Fe(OH)2 (s) + MnO4−(aq) → MnO2 (s) + Fe(OH)2 (s) In the balanced equation, 3 OH- are present on the product side. In the balanced equation, 5 molecules of water are present on the reagent side. In the balanced equation, 4 molecules of water are present on the product side. In the balanced equation, 5 molecules of water are present on the product side. In the balanced equation, 3 OH- are present on the reagent side. In the balanced equation, 4 molecules of water are present on the reagent side.arrow_forwardBalance the following redox reaction in the gas phase. Note that this reaction cannot be balanced with the half-reaction method and must be done by inspection. In the gas phase, H2 O (1) is used to balance oxygen atoms and H3 O+ and OH are not available for balancing purposes. ONLY integers should be used in your answer. NH3 (aq) + O2 (g) –→ NO2 (g) Enter your chemical notation herearrow_forwardPart A Consider the following unbalanced redox reaction. MnO, (aq) + Zn (s) → Mn²+(ag) + Zn²+(ag) Balance the equation: a MnOq (ag) + BH (ag) + y Zn (s) → 8 Mn2 (ag) + € H2O(1) + K Zn² (ag) Give your answer as an ordered set of numbers a, B, y, .. Use the least possible integers for the coefficients. Templates Symbols uado redo reset keyboard shortcuts help, α, β, γ, δ, ε, κ Submit Request Answer Part B Determine the volume of a 0.560 mol L-'KMN04 solution required to completely react with 2.65 g of Zn. Templates Symbols undo redo reset keyboard shortcuts help, V = mLarrow_forward
- Write balanced half-reactions for the following redox reaction: 3+ 3 Cl₂(g) +2 Cr³+ (aq) +7H₂O(1) → 6 Cl(aq) + Cr₂O² (aq) +14 H+ (aq) reduction: oxidation: ロ→ロ × S ?arrow_forwardGiven the reaction: Mg(s) + 2 HCl(aq) → MgCl2(aq) + H2(g) Which species is the reducing agent? HCI H2 MgCl2 Mgarrow_forwardIn the following UN-balanced redox reaction, which element is oxidized during the reaction? Cr(OH)3 (s) + Cu2+ (aq) → CrO42 (aq) + Cut (aq) H Cu O Cr 10000 Balance the following redox reaction under basic conditions: Cr(OH)3 (s) + Cu²+ (aq) → CrO42 (aq) + Cut (aq) How many electrons are transferred during the reaction? type your answer... electrons Balance the following redox reaction under basic conditions: Cr(OH)3 (s) + Cu²+ (aq) → CrO42 (aq) + Cut (aq) The hydroxide ion is on the reactant ✓side and and has a coefficient of 1arrow_forward
- Balance the following reaction in basic solution. Ag(s) + Zn (aq) 21 Ag,O(aq) + Zn(s) Fill in the coefficients for the balanced overall equation. Ag + Zn2- OH vZn + Ag, 0 + H,Oarrow_forwardBalance the reaction below in basic conditions and identify the oxidizing and reducing agents. Ag(s) + CN−(aq) + O2(g) → Ag(CN)2−(aq)arrow_forwardWhy is this equation not a redox reaction? Fe2(SO4)3(aq) + 3H2O(l)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY