
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
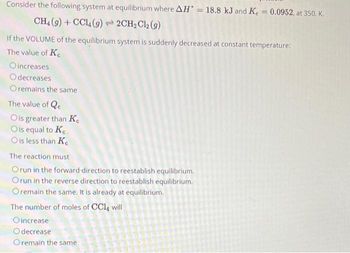
Transcribed Image Text:Consider the following system at equilibrium where AH = 18.8 kJ and K = 0.0952, at 350. K.
CH4 (9) + CCL (9)2CH₂Cl2 (9)
If the VOLUME of the equilibrium system is suddenly decreased at constant temperature:
The value of Ke
Oincreases
Odecreases
Oremains the same
The value of Qe
Ois greater than K
O is equal to Ke
O is less than Ke
The reaction must
Orun in the forward-direction to reestablish equilibrium.
Orun in the reverse direction to reestablish equilibrium.
O remain the same. It is already at equilibrium.
The number of moles of CCl4 will
Oincrease
Odecrease
Oremain the same
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The Haberprocess is exo thermic. Cthe formation of NH3 from its elements) a) wribe out the balanced Chemical equation (with subscripts) and include energy b) If you were to maximile product yiel, would increase or decrease heat you to the system? c) Explain what stress could have caused the changes the graph below N2 equilibrium reached NH, Concentration (mol/L)arrow_forwardFor the reaction CaO(s) + CO2(g) CaCO3(s), the value of AH is -178 kJ/mol. Using Le Chatelier's principle, which of the following changes to the system will shift the equilibrium to the right? i) Increasing the amount of CaO. ii) Decreasing the volume of the container. iii) Increasing the temperature. iv) Decreasing the temperature O A. only ii and iii OB. only i & ii OC. all of them OD. only i & iv OE. only i & iiiarrow_forwardConsider the following system at equilibrium where Ho = 18.8 kJ, and Kc = 9.52E-2, at 350 K: CH4(g) + CCl4(g) 2 CH2Cl2(g) If the VOLUME of the equilibrium system is suddenly decreased at constant temperature: whats the value of Kc The value of Q The reaction mustarrow_forward
- The chemical equation for an equilibrium is described below. kJ mol CaO (s) + CO2(g) = CaCO3(s) AH° Predict how the following changes would shift the equilibria. Be sure to start by writing the equilibrium (K) equation. =-176-arrow_forwardConsider the following system at equilibrium where H° = -111 kJ/mol, and Kc = 0.159, at 723 K. N2(g) + 3H2(g) 2NH3(g) If the TEMPERATURE on the equilibrium system is suddenly decreased: The value of Kc fill in the blank 1 A. increases. B. decreases. C. remains the same. The value of Q fill in the blank 2 A. is greater than Kc. B. is equal to Kc. C. is less than Kc. The reaction must: fill in the blank 3 A. run in the forward direction to reestablish equilibrium. B. run in the reverse direction to reestablish equilibrium. C. remain the same. It is already at equilibrium. The concentration of H2 will: fill in the blank 4 A. increase. B. decrease. C. remain the same.arrow_forward[Review Topics] [References] Use the References to access important values if needed for this question. Consider the following system at equilibrium where AH=-108 kJ, and K. 77.5, at 600 K. CO(g) + C,(g) COCI,(g) When 0.22 moles of Cl,(g) are added to the equilibrium system at constant temperature: The value of K The value of Qe K. The reaction must O run in the forward direction to restablish cquilibrium. O run in the reverse direction to restablish cquilibrium. O remain the same. It is already at cquilibrium. The concentration of CO will Submit Answer Retry Entire Group 1 more group attempt remainingarrow_forward
- Alert for not submit AI generated answer. I need unique and correct answer. Don't try to copy from anywhere. Do not give answer in image and hand writingarrow_forwardConsider the following equilibrium: N₂(g) + 3H₂(g)2NH3(g) AG = -34. KJ Now suppose a reaction vessel is filled with 3.40 atm of hydrogen (H₂) and 9.24 atm of ammonia system: Under these conditions, will the pressure of NH3 tend to rise or fall? Is it possible to reverse this tendency by adding N₂? In other words, if you said the pressure of NH3 will tend to rise, can that be changed to a tendency to fall by adding N₂? Similarly, if you said the pressure of NH3 will tend to fall, can that be changed to a tendency to rise by adding N₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of N₂ needed to reverse it. Round your answer to 2 significant digits. rise O fall yes Ono X (NH3) at 736. °C. Answer the following questions about this Sarrow_forwardWhen heated, carbon monoxide reacts with water to produce carbon dioxide and hydrogen. CO(g) + H2O(g) <--> CO2(g) + H2(g) +heat For each of the following changes at equilibrium, indicate whether the equilbrium shifts in the directions of the products, the reactants, or does not change: (a) decreasing the temperature (b) adding more H2(g) (c) removing some CO2(g) (d) adding more H2O(g) (e) decreasing the volume of the containerarrow_forward
- [References] Use the References to access important values if needed for this question. Consider the following system at equilibrium where AH° = 16.1 kJ, and Kc = 6.50 x 10-3, at 298 K: 2NOBr(g) 2NO(g) + Br₂(g) If the VOLUME on the equilibrium system is suddenly increased at constant temperature: The value of Ke Oincreases O decreases O remains the same The value of Qc O is greater than Ke O is equal to Ke O is less than K The reaction must run in the forward direction to reestablish equilibrium. Orun in the reverse direction to reestablish equilibrium. Oremain the same. It is already at equilibrium. The number of moles of Br2 will O increase O decrease O remain the same Show Hint F5 6 H F6 & 7 KI F7 * 8 DII F8 ( 9 DD F9 ) O F10 P F11 Previous + Next Save and Exit F12 deletearrow_forwardFor the equilibrium system below, which of the following would result in an increase in the quantity of H₂(g)? H₂(g) + 12(g) 2HI(g) + 65 kJ ос removing some I₂(g) and removing some HI(g) removing some HI(g) removing some 1₂(g) decreasing temperature removing some HI(g) and decreasing temperaturearrow_forwardConsider the following equilibrium: 2NO, (g)–N,04 (8) AGº= – = - 5.4 kJ Now suppose a reaction vessel is filled with 5.06 atm of dinitrogen tetroxide (N,0) at 400. °C. Answer the following questions about this system: 2 rise Under these conditions, will the pressure of N,0, tend to rise or fall? 2 4 fall Is it possible to reverse this tendency by adding NO,? In other words, if you said the pressure of N,0, will tend to rise, can yes that be changed to a tendency to fall by adding NO,? Similarly, if you no said the pres of N,0, will tend to fall, can that be changed to a 2 4 tendency to rise by adding NO,? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO, needed to reverse it. atm Round your answer to 2 significant digits.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY