
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
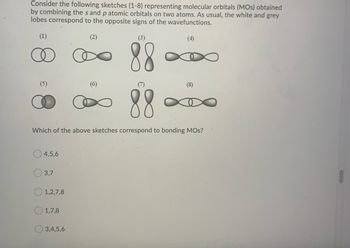
Transcribed Image Text:Consider the following sketches (1-8) representing molecular orbitals (MOs) obtained
by combining the sand p atomic orbitals on two atoms. As usual, the white and grey
lobes correspond to the opposite signs of the wavefunctions.
88
88
Which of the above sketches correspond to bonding MOS?
(5)
4,5,6
3,7
1,2,7,8
1,7,8
3,4,5,6
(8)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 9A.2 Write the valence bond wavefunction of the o bond in a C-H group of a molecule.arrow_forwardOne of the excited states of a C, molecule has the electron configuration: (01,)²(01,*)²(02,)²(02,*)'(T 2,)*(02,)'. What is the bond order of C, in this excited state? C, bond order in excited state: What is the bond order of C, in the ground state? C, bond order in ground state: How does the bond length in this excited state compare to that in the ground state? bond length (excited state) bond length (ground state)arrow_forwardthe order of decreasing size of the following ions is P3- > Cl- > K+ > Ca2+Select one:TrueFalsearrow_forward
- For the following two sets of atomic orbital combinations, identify the result of their combination as: (i) bonding, anti-bonding or non-bonding and (ii) if applicable, sigma or pi (a) (b) + + 88arrow_forwardDetermine which of the following species would have this electron configuration: (02s)²(02s*)²(π2p)*(02p)². A) O₂ B) SCI- C) C₂² D) CIF E) PS+arrow_forwardbnod slgnie bnod elduob d 7. Describe the change in hybridization (if any) for the antimony atom in the following reaction: SÜF5 + F- S6F6-arrow_forward
- AAX Welco Ch. 2 ← → C M Gmail 17°C Partly cloudy A histor app.101edu.co YouTube Maps Welcome to MyTCC W histor # 3 F3 E Word b Write b Answ $ Use the MO diagram (below) to calculate the bond order for NO+. F4 403 Forbidden: Ac... 4 R App ПР Op - 5 08 11 Ap ― -- QLD Gionic F6 A G Which Chem Question 13 of 50 F7 FB b Answ 4 0 19 8 FO b Answ ba. Pre G NO₂ G F10 O 1 IN 4 7 +/-arrow_forwardConsider the following sketches (1-8) representing molecular orbitals (MOS) obtained by combining the sand patomic orbitals on two atoms. As usual, the white and grey lobes correspond to the opposite signs of the wavefunctions. (1) 1, 5 3,7 88 Which of the above sketches correspond to bonding sigma MOS? 1,2 1, 2,8 (2) 4,5,6 (3) (8)arrow_forwardWhat is the ground state electron configuration for Be ? 1s 1s Incorrect What is the bond order for Be ? bond order: 1.5 Incorrectarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY