
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
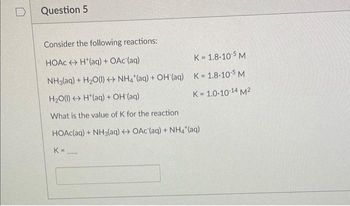
Transcribed Image Text:Question 5
Consider the following reactions:
HOẶC ↔ H*(aq) + OAc(aq)
K=1.8-10-5 M
NH3(aq) + H₂O(l) + NH4 (aq) + OH(aq) K=1.8-10-5 M
H₂O(l) H(aq) + OH (aq)
K = 1.0-10-14 M²
What is the value of K for the reaction
HOAc(aq) + NH3(aq) + OAc (aq) + NH4*(aq)
K=
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the following acid-base equilibria of weak acids in water, label the acid (A), the base (B), the conjugate acid (CA), and the conjugate base (CB). HCIO,(aq) + H,O(1) = H,O*(aq) + C1O, (aq) H₂CO3(aq) + H₂O(1) H₂O+ (aq) + HCO3(aq) H,O(1) +CH,NH (aq) = CH,NH,(aq) + H,O*(aq) O CH₂COOH(aq) + H₂O(1) ⇒ CH₂COO¯(aq) + H₂O+ (aq) O B Answer Bank CA A CBarrow_forwardWhat is the pH of a grapefruit that contains 0.007 M citric acid solution (C6H8O7)? C6H8O7(aq) + H2O(ℓ) ⇌ C6H7O7−(aq) + H3O+(aq) Ka = 7.5 x 10-4arrow_forwardFor each chemical reaction in the table below, decide whether the highlighted reactant is a Brønsted-Lowry acid, a Brønsted-Lowry base, or neither, highlighted reactant reaction Bronsted-Lowry Bronsted-Lowry neither acid base NH (aq) + OH (aq) → NH,(aq) + H,O(1) NH (aq) + OH (aq)→ NH,(aq) + H,O() NH,(aq) + H,O(1) – NH(aq) + OH (aq) NH,(aq) + H,O() - NH(aq) + OH (aq) Continue Submit O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use I Privacarrow_forward
- = O ACIDS AND BASES Identifying Bronsted-Lowry acids and bases For each chemical reaction in the table below, decide whether the highlighted reactant is a Brønsted-Lowry acid, a Brønsted-Lowry base, or neither. reaction NH(aq) + OH (aq) → NH3(aq) + H₂O(1) NH3(aq) + H₂O(1)→ NH(aq) + OH(aq) NH4(aq) + OH (aq) ► NH3(aq) + H₂O(1) NH3(aq) + H₂O(1)→ NH(aq) + OH(aq) 1 highlighted reactant Bronsted-Lowry Bronsted-Lowry acid base O O O O O neither O O 0 0/5 Oarrow_forward(a) Using the expression Ka=[H+][A−]/[HA], explain how to determine which solution has the lower pH, 0.10MHF(aq) or 0.10MHC2H3O2(aq). Do not perform any numerical calculations. (b) Which solution has a higher percent ionization of the acid, a 0.10M solution of HC2H3O2(aq) or a 0.010M solution of HC2H3O2(aq) ? Justify your answer including the calculation of percent ionization for each solution.arrow_forwardCalculate the K of A" given HA(aq) + H2O(l) = H₂O† (aq) + A¯¯(aq) K = 5.90×108arrow_forward
- Identify the reactant that is a Brønsted-Lowry acid and the reactant that is a Brønsted-Lowry base in each of the following: Part C 2- CO3² (aq) + H₂O = HCO3(aq) + OH-(aq) H₂O is the acid (proton donor); CO3² is the base (proton acceptor). H₂O is the acid (proton acceptor); CO² is the base (proton donor). O CO3²- is the acid (proton acceptor); H₂O is the base (proton donor). O CO² is the acid (proton donor); H₂O is the base (proton acceptor). Submit Part B Request Answer H₂SO4 (aq) + H₂O(1)→ H3O+ (aq) + HSO4 (aq) O H₂O is the acid (proton donor); H₂SO4 is the base (proton acceptor). O H₂SO4 is the acid (proton acceptor); H₂O is the base (proton donor). O H₂SO4 is the acid (proton donor); H₂O is the base (proton acceptor). O H₂O is the acid (proton acceptor); H₂SO4 is the base (proton donor). CH3-COO (aq) + H3O+ (aq) = H₂O(1) + CH3-COOH(aq) CH3-COO is the acid (proton acceptor); H3O+ is the base (proton donor). O H3O+ is the acid (proton donor); CH3-COO is the base (proton acceptor). O…arrow_forwardHA is a weak acid. Select the correct dropdown value to correctly complete the incomplete concentration table below being used to determine the equilibrium concentrations of HA, H3O*, and A¯ of a 0.1300 M HA solution that has a pH of 3.60 at 25°C. HA(aq) + H,0(1) = H,0^(aq) + A-(aq) Initial 0.1300 Change Equilibrium (b) (a] The value for a is [ Select ] The value for b is [ Select ] What is the Ka of this weak acid? [ Select]arrow_forwardWhat is the pOH for a solution at 25 °C that has a H3O+ concentration of 6.57 ×10-6 M? A 34.1 % (NH4 )2SO4 (molar mass = 132.1 g mol−1) has a density of 1.15 g mL−1. What is the molarity of this solution? (the answer should be entered with 3 significant figures; do not enter units; give answer in normal notation--examples include 1.23 and 120. and -123 and 123. and 12.3) Determine the boiling point of a solution that contains 78.5 g of compound W (molar mass = 132.5 g mol–1) dissolved in 1088.6 g benzene (C6H6; molar mass = 84.156 g mol–1; Kb = 2.53 °C m–1; boiling point of pure benzene = 80.1 °C). (the answer should be entered with 3 significant figures; do not enter units; give answer in normal notation--examples include 1.23 and 120. and -123 and 123. and 12.3) NEED HELP ASAP NO WORK NEEDEDarrow_forward
- 5) Find the concentration of H30*(aq) in a 1.75 M solution of lactic acid, HC3H5O3, at 25°C. Ka= 1.38 x 10*. 6) Write the equilibrium expression for the ionization of HOI, and calculate the concentration of HOI(aq) in solution if [H3O*]=2.3 x 10° M and pKa = 10.7 at 25°C.arrow_forwardWhat is [OH-] in a solution of 1.25 M NH3 and 0.85 M NHANO3? NH3 (aq) + H20 (1) = NH4+ (aq) + OH- (aq) Kp=1.8x10-5 Write your answer with 2 significant digits.arrow_forwardAt 25 oC, Ammonia is a weak base that reacts with water according to this equation: NH3(aq) + H2O(aq) ⇌ NH4+(aq) + OH−(aq) Briefly explain how the equilibrium will shift (to get back to equilibrium) if the following perturbations are made to the system: (a) Addition of HCl (b) Addition of NaOH (c) Addition of NH4Clarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY