
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
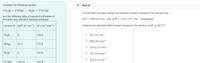
Transcribed Image Text:Consider the following reaction:
Part D
2 H2(g) + 2 NO(g) →
N2(g) + 2 H20(g)
The standard enthalpy change and standard entropy change for the reaction are:
and the following table of standard enthalpies of
formation and standard absolute entropies:
A;H° = -666.2 kJ mol-1 and A,S° = -114.0 J K-1 mol-1, respectively.
compound AH (kJ mol-1) s° (J K-1 mol-1)
Compute the standard Gibbs energy change for the reaction, AG°, at 32.0 °C.
|H2(g)
130.8
301.4 kJ mol-1
2981.8 kJ mol-1
NO(g)
91.3
210.8
-4314.2 kJ mol-1
N2(g)
191.6
O -701.0 kJ mol-1
O -631.4 kJ mol-1
H,O(g)
-241.8
188.8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use the standard reaction enthalpies given below to determine AH°rxn for the following reaction: Given: P4(g) + 10 Cl2(g) PC15(s) -> ->> 4 PC15(s) PC13(g) + Cl2(g) P4(g) + 6 Cl2(g) → 4 PC13(g) AH°rxn = ? AH°rxn = +144 kJ AH°rxn-1 = -1298 KJarrow_forward4. Calculate the standard heat of formation of calcium carbide (CaC) from its elements in kJ/mol using the following thermochemical equations. (hint come up with the overall equation first) Ca(s) + 2H₂O ⇒ Ca(OH)2(s) + H₂(g) 2C(s) + O2(g) → 2CO CaO + H₂O⇒ Ca(OH)2() 2H2(g) + O2(g) → 2H₂O) CaO) + 3C,,→ CaCs) + CO ΔΗ°= - 414.79 kJ ΔΗ°= - 221.00 kJ ΔΗ°= - 65.19 kJ ΔΗ°= - 571.80 kJ AH°= + 462.30 kJ 1arrow_forward7) Calculate the change in internal energy (AE) for a system that is giving off 25.0 kJ of heat and is changing from 12.00 L to 6.00 L in volume at 1.50 atm pressure. (Remember that 101.3 J = 1 L'atm) A) +25.9 kJ B) -16.0 kJ C) -25.9 kJ D) -24.1 kJ E) 937 kJ Answer: D Diff: 2 Page Ref: 6.4arrow_forward
- Consider this reaction: C6H12O6 + 602 à 6CO2 + 6H₂O. What is the standard enthalpy AH°rxn given the standard molar enthalpies of the substances below? Substance C6H12O6 02 CO₂ H₂O AHᵒf (kJ/mol) -1274.4 0 -393.45 -241.8arrow_forward55. Does the standard enthalpy of formation of H2O(g) differ from ΔH° for the reaction 2H2(g)+O2(g)⟶2H2O(g)?arrow_forward1,2,3 pleasearrow_forward
- 5. Use standard heats of formation and the following equation to determine the enthalpy of formation of C₂H() ΔΗ, = -3127 kJ 2C₂H6(g) +70₂(g) ⇒ 4CO2(g) + 6H₂O)arrow_forwardFor the reaction given below: A2(g) + B2(g) --> 2AB(g) the change in internal energy is 2.5 KiloJoules at 298.15 Kelvins. What would be the corresponding change in enthalpy (in KiloJoules) for the reaction?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY