
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
If my first arrows are correct, then what would the second arrows look like?
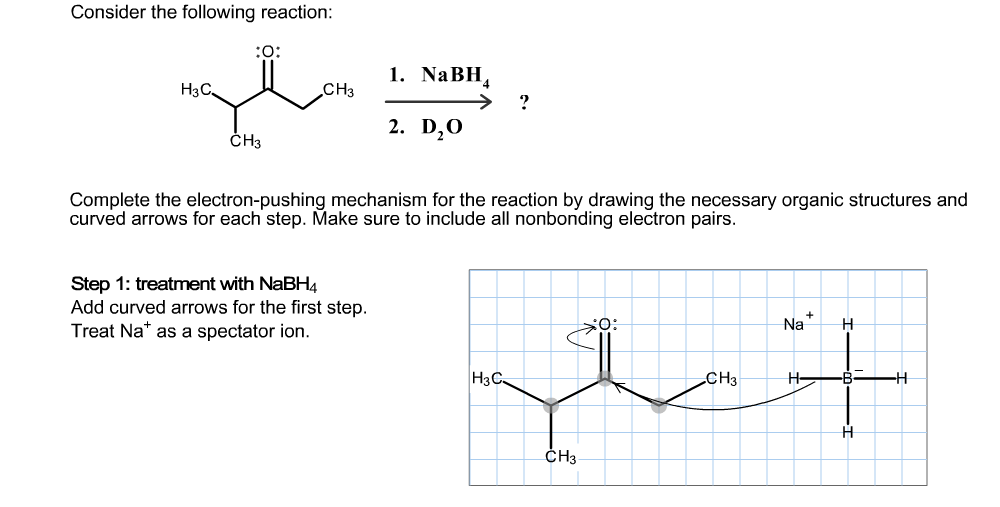
Transcribed Image Text:Consider the following reaction:
O:
1. NaBH
H3C
CНз
2. D20
CH3
Complete the electron-pushing mechanism for the reaction by drawing the necessary organic structures and
curved arrows for each step. Make sure to include all nonbonding electron pairs.
Step 1: treatment with NaBH4
Add curved arrows for the first step.
Treat Na as a spectator ion.
Na
-В-
НзС.
Cнз
H
CHз
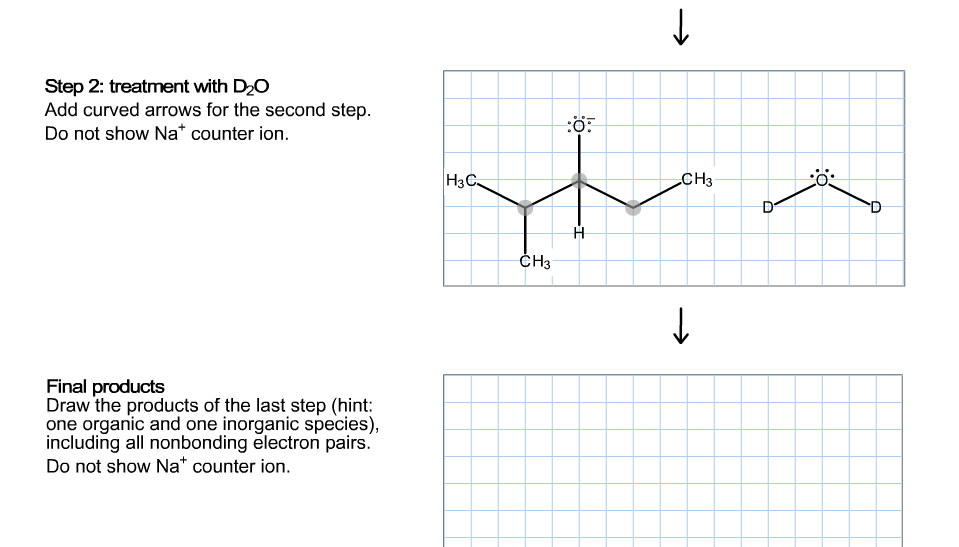
Transcribed Image Text:Step 2: treatment with D2O
Add curved arrows for the second step
Do not show Na* counter ion
:ö
|НзС-
CH3
СHз
Final products
Draw the products of the last step (hint:
one organic and one inorganic species),
including all nonbonding electron pairs.
Do not show Na* counter ion
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What effect does the ozone layer have on the EM waves from the sun? What is currently threatening the ozone layer? Describe the greenhouse effect occurring in Earth's atmosphere? Iarrow_forwardWe are talking about the extraction of fats from potato chips.arrow_forwardQ/2 Predict the maximum wavelength for: Q3/ Explain how the polar solvent increase the maximum wavelength value. Q4/ when an organic molecule is exposed to ultraviolet rays, what happens?arrow_forward
- Pls help ASAP. Pls show all work and calculations.arrow_forward15. a. Draw two constitutional isomers with the formula CaHsBrCl ( Isomer 1 Isomer 2 b. Describe ALL the intermolecular forces isomer 1 can participate in 16. For the molecule below, OH (a) Circle all functional groups present. (b) Label two of the functional groups next to where you circled them 3arrow_forwardQuestion 2) A polar compound will have a (smaller/larger) Rf when a polar stationary phase is usedarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY