
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
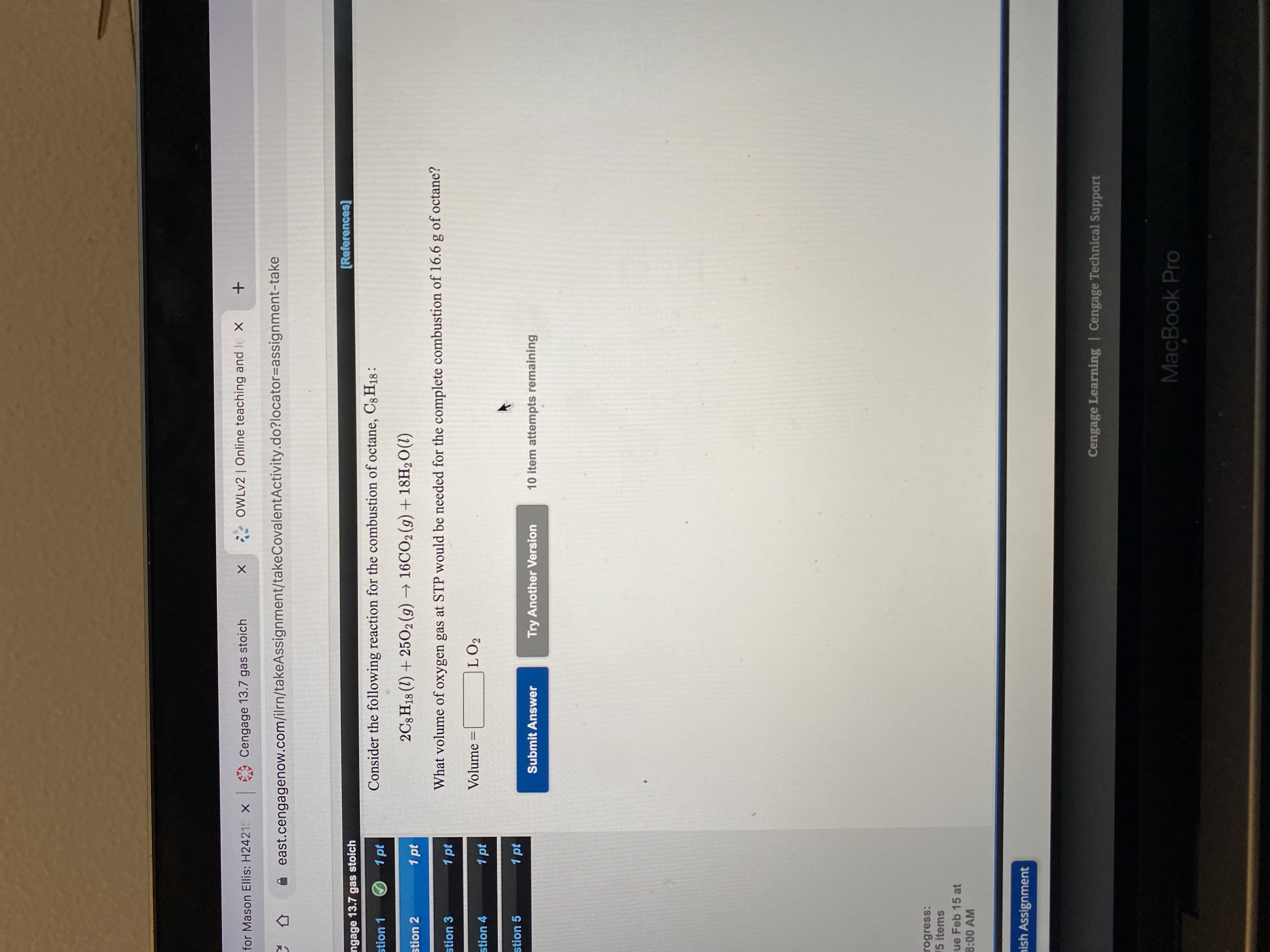
Transcribed Image Text:Consider the following reaction for the combustion of octane, C8H18:
2C3 H18 (1) + 2502(9) → 16CO2 (9) + 18H2O(1)
What volume of oxygen gas at STP would be needed for the complete combustion of 16.6 g of octane?
Volume
LO2
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- To the nearest 0.1 g, how many grams of a candle wax from a different brand of candle can be burned in 5-gallon container sealed with air? Assumptions (may or may not be needed): Complete combustion to CO2 and H2O; Candle wax reacts in a ratio of 1 mol wax: 40 moles O2; MW (wax) = 370 g/mol, Air = 21% oxygen, R= 0.082 L.atm.K-1.mol-1, 1 gallon = 3.785 L, T=278 K, P = 1 atmarrow_forwarddetermine 1) the limiting reagent, 2) the moles of each reactant left over, and 3) volume (in L) of any gaseous products at STP. Combustion of 9.112 X 1023 molecules of methane (CH4) with 75.0 L of O2 at 1.0 atm and 299.15 K (assume any water produced is liquid).arrow_forwardWhat is the mass in grams of Al that were reacted with excess HCl if 3.46 L of hydrogen gas were collected at STP in the following reaction? 2 Al (s) + 6 HCl (aq) = AlCl3 (aq) + 3H2 (g)arrow_forward
- Consider the following reaction for the combustion of octane, C8H18: 2 C8H18(l) + 25 O2(g) → 16 CO2(g) + 18 H20(l) What volume of oxygen gas at STP would be needed for the complete combustion of 33.0 g of octane?arrow_forwardCHEM 1 Please solve correctly and give the complete solution.arrow_forwardConsider the complete combustion of glucose (C6H12O6) with O2 and calculate the moles of CO2produced when 1.02 g of glucose is reacted with 25 mL of O2 at body temperature (37 ºC) and 0.970 atm. C6H12O6 + 6 O2 (g) → 6 CO2 (g) + 6 H2O a. 0.95 b. 0.0022 c. 9.53 x 10-4 d. 6.33 x 10-2 e. 5.67 x 10-3arrow_forward
- How many grams of NaClO3 were decomposed?arrow_forwardHow many grams of Magnesium Oxide form when 14.8L of oxygen gas, measured in STP, completely reacts with Magnesium metal in accordance with the following reaction : 2MgO (s) + O2 (g) = 2MgO (s)arrow_forwardConsider the reaction shown below: 2 HCl (aq) + CaCO3 (s) ⟶ CO2 (g) + CaCl2 (aq) + H2O (l) How many grams of CaCO3 would need to be consumed to fill a 908.001 mL balloon with CO2 gas, assuming a constant temperature of 21.7 oC and constant pressure of 0.958 atm? Express your answer in units of grams using at least three significant figures.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY