
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
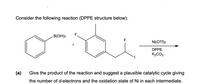
Transcribed Image Text:Consider the following reaction (DPPE structure below):
F.
B(OH)2
F
Ni(OTf)2
+
DPPE
K2CO3
(а)
Give the product of the reaction and suggest a plausible catalytic cycle giving
the number of d-electrons and the oxidation state of Ni in each intermediate.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Kk.351arrow_forwardDiscuss the binding mode and geometry of the imido and cyclopentadienyl ligands in the tungsten complex below.arrow_forwardTriphenylphosphine as a free ligand shows a 3¹P NMR signal at -4 ppm. Cis-Pt(PPh3)2Cl2 shows a single resonance at +12 ppm. Cis-Pd(PPh3)2Cl2 shows a single resonance at +24 ppm. Give a plausible explanation as to why the 31P resonance in the Pd complex is shifted to a more deshielded position than in the Pt complex. (Hint: check the periodic table)arrow_forward
- 3. a. (i) Starting from K₂[PtC14], plan synthetic routes to the two complexes A and B shown below, using your knowledge of the trans effect. Formulate each step by drawing structures of each complex and give a full explanation for your choices. Make sure to assign the correct charges to each complex in each case. 7- 7- (ii) NCS NH₂ -Pt-Br CI NO₂ CI-Pt-OH CO B Write a detailed discussion of the two factors that may contribute to the overall trans-directing ability of a particular ligand, considering thermodynamic and kinetic aspects. Use appropriate examples and orbital sketches to support your explanations.arrow_forwardShow Calculations and Formulas. What is the energetic favorability of A? (A) MnO2 reduction by sulfide yielding S0 Compared to the energetic favorability of B? (B) energetic favorability of MnO2 reduction by sulfide yielding SO42-.arrow_forwardConsider the reaction between dissolved Fe2+ and elemental sulfur (S0) to form hematite (Fe2O3) and sulfide a) Identify the oxidant and reductant in this reaction, determine half-reactions, and balance thereaction. b) Is this reaction thermodynamically favorable? c) Would you expect HS- to react readily with Fe2O3 (i.e. the reverse of the reaction)?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY