
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Consider the following reaction and predict the new
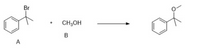
Transcribed Image Text:Br
CH;OH
A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Some measurements of the initial rate of a certain reaction are given in the table below. [N2] H2 initial rate of reaction 0.802M 1.70M 77.0M/s 0.802M 0.476M 21.6M/s 1.77M| 1.70M 375.M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = k || x10 k =arrow_forwardSome measurements of the initial rate of a certain reaction are given in the table below. [N2] H2 initial rate of reaction 1.02M 1.73М 0.0437M/s 1.02M |6.60М 0.167 M/s 0.333 M 1.73М 0.00466M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = k k =arrow_forwardDetermine the rate of the last set of reaction conditions. [A]o [B]o Rate, M/s 0.370 0.311 2.10e-4 0.596 0.311 2.10e-4 0.370 0.589 2.89e-4 0.596 0.501 ?arrow_forward
- The rate of reaction was measured during a chemical reaction. After the first 3 seconds, the rate of reaction was 1.8 x10−6 M/s. Which of the following would you expect after another 3 seconds? The rate would be lower, and the concentration of products would be higher. The rate would be lower, and the concentration of products would be lower. The rate would be higher, and the concentration of products would be higher. The rate would be higher, and the concentration of products would be lowerarrow_forwardWhich statement is INCORRECT with regard to kinetics? Each 10° C rise in temperature approximately doubles the rate of a reaction. The rate is expressed as the change in concentration per the change in time. The rate of reaction is always expressed as a positive value. The presence of a catalyst decreases the rate of reaction by lowering the E, of the reaction. The effect that changing the concentration of a reactent has on the rate of a reaction must be determined experimentally.arrow_forwardFor a given reaction, how will the rate constant (k) change with temperature? The rate constant will be the same at all temperatures. The rate constant will increase as temperature increases. The rate constant will decrease as temperature increases. How the rate constant changes will depend on what the specific reaction is.arrow_forward
- Classify the actions based on how they could affect a reaction rate. Rate increases Rate decreases Rate is unaffected Answer Bank decrease the concentration of a reactant decrease the surface area of a solid reactant increase the temperature add a catalystarrow_forwardSome measurements of the initial rate of a certain reaction are given in the table below. [N-] [H.] initial rate of reaction 2.27 M 1.81 M 16.0M/s 2.27 M 0.429 M 0.899 M/s 9.19 M 1.81 M 262. M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol.arrow_forwardAt a given temperature, a reaction has a rate constant of 5.83 x 10 -5 s -1. If the starting concentration is 2.65 Molar, the initial instantaneous rate will be At a given temperature, a reaction has a rate constant of 5.83 x 10 -5 s -1. If the starting concentration is 2.65 Molar, the initial instantaneous rate will be 6.91 x 10 -7 M/s 2.00 M/s 4.28 x 10 -1 M/s 0.962 M/s 7.15 x 10 -1 M/s 8.93 x 10 -3 M/s 1.54 x 10 -4 M/s 1.56 M/sarrow_forward
- The rate of a reaction is how quickly the reaction goes to completion. If two reactions have the same amount of reactant, increasing the rate does not increase the amount of product produced, it simply reduces the time that it takes to make the product. Using the internet , research the two factors below that affect the rate of a chemical reaction. 1)Temperature ( add an example and be very detailed add graphs as well) 2) Presence of a catalyst. ( add an example and be very detailed add graphs as well) Your work should be written in complete sentences, and assume your audience is grade 11 chemistry students who do not understand this unit. Make sure all research is put into your own words!arrow_forward8. Smelling salt decomposes spontaneously at room temperature by the equation below. A student places a 10.00-g sample into a 1-liter sealed rigid vessel. What measurements should the student make to determine the rate of this reaction?(NH4)2CO3(s)→2NH3(g)+H2O(g)+CO2(g) a. change in mass and temperature b. temperature and time c. change in mass and time d. change in pressure and timearrow_forwardSome measurements of the initial rate of a certain reaction are given in the table below. [N:] H2 initial rate of reaction 0.355M 2.28M 17.0M/s 0.355M 0.704M 1.62M/s 0.0958M 2.28M 4.59M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = k|| x10 k =arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY