
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
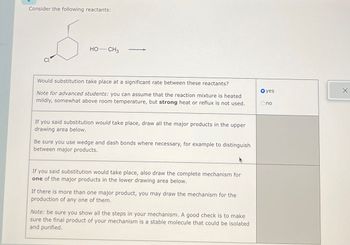
Transcribed Image Text:Consider the following reactants:
HO-CH3
Would substitution take place at a significant rate between these reactants?
Note for advanced students: you can assume that the reaction mixture is heated
mildly, somewhat above room temperature, but strong heat or reflux is not used.
If you said substitution would take place, draw all the major products in the upper
drawing area below.
Be sure you use wedge and dash bonds where necessary, for example to distinguish
between major products.
If you said substitution would take place, also draw the complete mechanism for
one of the major products in the lower drawing area below.
If there is more than one major product, you may draw the mechanism for the
production of any one of them.
Note: be sure you show all the steps in your mechanism. A good check is to make
sure the final product of your mechanism is a stable molecule that could be isolated
and purified.
yes
no
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1.Draw a structural formula for the more stable carbocation intermediate formed in the reaction shown. 2.Draw a structural formula for the major organic product of the reaction shown.arrow_forwardDraw an appropriate alcohol reactant on the left-hand side of this organic reaction. Also, if any additional major products will be formed, add them to the right-hand side of the reaction. If there's no alcohol reactant that can produce these products in one step in good yield, check the box under the drawing area instead. + ⑤ Click and drag to start drawing a structure. CrO3 H2SO4 H₂O Harrow_forwardNucleophilic Substitution and Elimination. A Worksheet Lab. Pre-Lab 1. Identify the following organic transformations. Are they "substitutions" or "additions" or "eliminations"? Write formulas that clearly illustrate you selection. Pay attention to the organic part of the transformations. Example. Br ultraviolet light HBr + Br₂ formulas: CsH12 Br2 CsH11Br HBr This is a "substitution" reaction. H has been replaced (substituted) by Brarrow_forward
- Draw a structural formula of an alkene or alkenes (if more than one) that undergo acid-catalyzed hydration and without rearrangement give 1-methylcyclohexanol as the major product. You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. ● If more than one structure fits the description, draw them all. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate structures with + signs from the drop-down menu. ● ● ✓ ? ChemDoodleⓇ n [ ]#arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps.arrow_forwardi need the answer quicklyarrow_forward
- What is the missing reactant in this organic reaction? NH2 +R + H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed.arrow_forwardQ4. Alkyl halides are good at doing substitution (SN2/SN1) and elimination (E1/E2) reactions because the halide (X-) is a good LG. Alcohols on the other hand are not suitable for these reactions since hydroxide (¯OH) is a poor LG. However, an OH can be converted into a OTs (tosyl group) by reacting an alcohol with TsCl in presence of a base. An OTs is a good LG, and this allows us to indirectly (via OTs) use alcohols in substitution (SN2/SN1) and elimination (E1/E2) reactions. (4) OH TsCl NEt3 OTS Tosyl ester NaCN DMSO + HNEt3 Determine the major/minor products CH3OH heat OTS Tosyl ester TsCl = NaOCH3 CH3OH DBU Ś=0 CIarrow_forward29 minutes, 42 seconds. Question Completion Status: A Moving to another question will save this response. Question 15 What is not an expected product of the following allylic substitution reaction? NBS, hv Br Br Compounds II and II Compound II only O Compound I only O Compound II only A Moving to another question will save this response O O Carrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting structure, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Then draw any missing organic intermediates or products for this reaction. Include all lone pairs in the structures. Ignore inorganic byproducts and counterions. H 0:0- HsC H Select to Add Arrows H HaC Select to Add Arrows CH₂OH, H CH3OH, H* CH3OH, H* HaC H Select to Add Arrows 1 CH3OH, H+ HaC H H Select to Add Arrows Iarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. CI H H Select to Add Arrows Q H H C → :CI:O Select to Add Arrows H H H HH :CI:0 Select to Add Arrowsarrow_forwardDraw the structural formula for all the alkenes with the indicated molecular formula that, without undergoing a rearrangement, produce the compound shown as a major product. Br. C7H12 Br2/H₂O OH . You do not have to consider stereochemistry. • If more than one structure fits the description, draw them all. ⚫ Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate structures with + signs from the drop-down menu. 0 ? F ChemDoodle Previous Nextarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY