
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
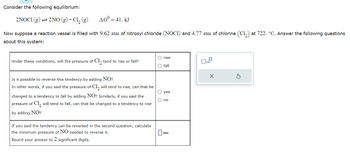
Transcribed Image Text:Consider the following equilibrium:
2NOC1 (g) → 2NO(g) + Cl₂ (g)
AG = 41. KJ
Now suppose a reaction vessel is filled with 9.62 atm of nitrosyl chloride (NOCI) and 4.77 atm of chlorine (C₁₂) at 722. °C. Answer the following questions
about this system:
Under these conditions, will the pressure of Cl₂ tend to rise or fall?
Is it possible to reverse this tendency by adding NO?
In other words, if you said the pressure of Cl₂ will tend to rise, can that be
changed to a tendency to fall by adding NO? Similarly, if you said the
pressure of C1₂ will tend to fall, can that be changed to a tendency to rise
by adding NO?
If you said the tendency can be reversed in the second question, calculate
the minimum pressure of NO needed to reverse it.
Round your answer to 2 significant digits.
O rise
O fall
O yes
O no
0₂
atm
0
x10
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studying this reaction fills a 100. L tank with 9.9 mol of ammonia gas, and when the mixture has come to equilibrium measures the amount of nitrogen gas to be 0.50 mol. Calculate the concentration equilibrium constant for the decomposition of ammonia at the final temperature of the mixture. Round your answer to 2 significan digits. K = 0arrow_forwardA reaction vessel contains an equilibrium mixture of SO2, O2, and SO3. The reaction proceeds such that: 2S0, (g) +0,(g) = 2SO3 (g)arrow_forwardConsider the equilibrium system described by the chemical reaction below. If the partial pressures at equilibrium of NO, Cl2, and NOCI are 0.095 atm, 0.171 atm, and 0.28 atm, respectively, in a reaction vessel of 7.00 L at 500 K, what is the value of Kp for this reaction? 2 NO(g) + Cl2(g) = 2 NOCI(g)arrow_forward
- Consider the following reaction that takes place at 1200 °C: H, (g) + CI, (g) + 2HCI (g) Kc = 2.5x10 Hydrogen gas and chlorine gas were added to a reaction vessel at partial temperatures of 2.00 atm and 1.00 atm, respectively. Calculate the total pressure in the flask at equilibrium.arrow_forwardConsider the following equilibrium: 2NOC1 (g) → 2NO(g) + Cl₂ (g) AG = 41. kJ Now suppose a reaction vessel is filled with 8.30 atm of nitrosyl chloride (NOC1) and 5.80 atm of chlorine (C1₂) at 878. °C. Answer the following questions about this system: Under these conditions, will the pressure of Cl₂ tend to rise or fall? Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of Cl₂ will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if you said the pressure of Cl₂ will tend to fall, can that be changed to a tendency to rise by adding NO? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. оо rise fall yes no 0 atm x10 X Śarrow_forwardAmmonia has been studied as an alternative "clean" fuel for internal combustion engines, since its reaction with oxygen produces only nitrogen and water vapor, and in the liquid form it is easily transported. An industrial chemist studying this reaction fills a 1.5 L flask with 1.7 atm of ammonia gas and 2.1 atm of oxygen gas, and when the mixture has come to equilibrium measures the partial pressure of water vapor to be 0.77 atm. Calculate the pressure equilibrium constant for the combustion of ammonia at the final temperature of the mixture. Round your answer to 2 significant digits. K = ☐ P x10arrow_forward
- Consider the following equilibrium: N,0, (g) = 2NO, (3) AG' = 5.4 kJ Now suppose a reaction vessel is filled with 0.496 atm of nitrogen dioxide (NO,) at 137. °C. Answer the following questions about this system: O rise Under these conditions, will the pressure of NO, tend to rise or fall? O fall Is it possible to reverse this tendency by adding N,O,? In other words, if you said the pressure of NO, will tend to rise, can that O yes be changed to a tendency to fall by adding N,04? Similarly, if you said O no the pressure of NO, will tend to fall, can that be changed to a tendency to rise by adding N,0,? If you said the tendency can be reversed in the second question, calculate the minimum pressure of N,0, needed to reverse it. O atm Round your answer to 2 significant digits.arrow_forwardSulfuryl chloride, SO2Cl2, is a highly reactive gaseous compound. When heated, it decomposes into sulfur dioxide and chlorine. A sample of 3.509 grams of SO2Cl2 is placed in an evacuated 1.00 L bulb and the temperature is raised to 102.0 °C. When the system has come to equilibrium at 102.0 °C, the total pressure in the bulb is found to be 1.43 atm. What is the Kp? (Use ICE table)arrow_forwardConsider the following equilibrium: 2NH3(g) = N₂ (g) + 3H₂(g) 2 AG = 34. kJ Now suppose a reaction vessel is filled with 7.87 atm of ammonia (NH3) and 1.17 atm of hydrogen (H₂) at 448. °C. Answer the following questions about this system: Under these conditions, will the pressure of NH3 tend to rise or fall? Is it possible to reverse this tendency by adding N₂? In other words, if you said the pressure of NH3 will tend to rise, can that be changed to a tendency to fall by adding N₂? Similarly, if you said the pressure of NH3 will tend to fall, can that be changed to a tendency to rise by adding N₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of N₂ needed to reverse it. Round your answer to 2 significant digits. rise fall yes no atm x10 X 5arrow_forward
- For the reaction 2 A(g) = B(g) + 3 C(g), we begin with only pure A(g) and the total pressure is 8.00 atm. We attain equilibrium. At equilibrium, the total pressure is 12.00 atm. What is the value of ∆G◦ for this reaction? The temperature is 25.0 ◦C throughout.arrow_forwarddemonstrate an understanding of the concept of dynamic equilibrium and the variables that cause shifts in the equilibrium of chemical systems.arrow_forwardConsider the following equilibrium: 2NO₂(g) → N₂O4 (g) AG = -5.4 kJ Now suppose a reaction vessel is filled with 0.202 atm of nitrogen dioxide (NO₂) at 504. °C. A Under these conditions, will the pressure of NO₂ tend to rise or fall? Is it possible to reverse this tendency by adding N₂O4? In other words, if you said the pressure of NO₂ will tend to rise, can that be changed to a tendency to fall by adding N₂O4? Similarly, if you said the pressure of NO₂ will tend to fall, can that be changed to a tendency to rise by adding N₂O4? If you said the tendency can be reversed in the second question, calculate the minimum pressure of N₂O needed to reverse it. V Round your answer to 2 significant digits. Orise fall O yes no atm 0arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY