
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
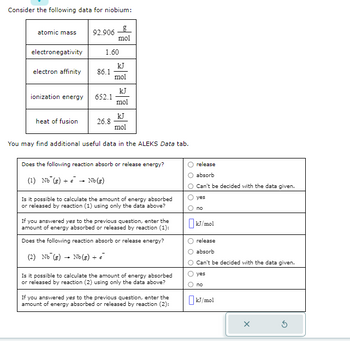
Transcribed Image Text:Consider the following data for niobium:
atomic mass 92.906
electronegativity
electron affinity
ionization energy
heat of fusion
1.60
86.1
652.1
g
mol
26.8
kJ
mol
kJ
mol
kJ
mol
You may find additional useful data in the ALEKS Data tab.
Does the following reaction absorb or release energy?
(1) Nb (g) + → Nb (g)
Is it possible to calculate the amount of energy absorbed
or released by reaction (1) using only the data above?
If you answered yes to the previous question, enter the
amount of energy absorbed or released by reaction (1):
Does the following reaction absorb or release energy?
(2) Nb (g) Nb (g) + €
Is it possible to calculate the amount of energy absorbed
or released by reaction (2) using only the data above?
If you answered yes to the previous question, enter the
amount of energy absorbed or released by reaction (2):
release
absorb
Can't be decided with the data given.
yes
no
☐kJ/mol
release
absorb
Can't be decided with the data given.
yes
no
☐kJ/mol
X
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many electrons are in the most likely ion formed from an atom located in group 5A and period 3? O 12 O 16 18arrow_forwardIn a reaction between water and lithium, 82.14 J of energy was released. The molar enthalpy change with respect to lithium for this reaction is +59.00 kJ/mol Li. What mass of Li reacted?arrow_forwardAn element has the following electron configuration, [Ar]3d104s²4p3. This element is a _-- O transition metal O metal noble gas nonmetal O metalloidarrow_forward
- The energy required to remove the second electron from a gaseous lithium in the gound state is 1.212 x 10^-17 joules. What is the value of IE2 for lithiumarrow_forwardConsider the following data for indium: atomic mass electronegativity ionization energy 114.82 electron affinity 28.9 heat of fusion 1.78 558.3 mol 3.26 kJ mol kJ mol kJ mol Does the following reaction absorb or release energy? (1) In (g) + e In* (g) Is it possible to calculate the amount of energy absorbed or released by reaction (1) using only the data above? If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (1): Does the following reaction absorb or release energy? (2) In (g) + e In (g) Is it possible to calculate the amount of energy absorbed or released by reaction (2) using only the data above? If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (2): O release O absorb O Can't be decided with the data given. O yes O no ☐kJ/mol O release O absorb O Can't be decided with the data given. O yes O no kJ/molarrow_forwardWhich of the following atoms has the greatest difference between IE3 and IE4? N Al P Barrow_forward
- esc 19 20 21 18 22 23 24 25 26 27 28 29 For each atom in the table below, write down the subshell from which an electron would have to be removed to make a +1 cation, and the subshell to which an electron would have to be added to make a -1 anion. The first row has been completed for you. atom subshell from which electron removed to form +1 cation subshell to which electron added to form -1 anion H 1s 1s 0 0 Submit Assignr 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Access Grey Black and B....pdf brown:green.jpeg DII F8 F9 F10 F11 Ti Na Kr Continue Two Column N....pages F1 ! F2 7 10 Kindergarten O....docx 80 F3 $ Q F4 X Grey Black and B....pdf % 0 10 F5 A ? MacBook Air F6 & F7 * Carrow_forwardMISSED THIS? Read Section 9.7. You can click on the Review link to access the section in your eText. Consider the following set of ionization energies: IE₁ = 1000 kJ/mol IE2 = 2250 kJ/mol IE3 3360 kJ/mol IE4 = 4560 kJ/mol IE5 7010 kJ/mol IE6 8500 kJ/mol IE7 = 27100 kJ/mol = = Part A To which third-period element do these ionization values belong? Spell out the full name of the element. Submit Provide Feedback Request Answerarrow_forwardConsider the following data for nickel: atomic mass g 58.693 mol electronegativity 1.91 electron affinity kJ 112. mol kJ 737.1 mol ionization energy kJ 17.2 mol heat of fusion You may find additional useful data in the ALEKS Data tab. Does the following reaction absorb or release energy? release absorb (1) Ni (g) Ni (g) + e O Can't be decided with the data given. O yes Is it possible to calculate the amount of energy absorbed or released by reaction (1) using only the data above? O no Explanation Check © 2022 McGraw Hill LLC. All Righarrow_forward
- Calculate the kinetic energy of an electron moving at a speed of 4.45 x 105 m/s (see the hint for electron properties). x 10arrow_forwardConsider the following data for cobalt: atomic mass 58.933 electronegativity electron affinity ionization energy heat of fusion 1 63.7 (2) Co (g) 1.88 760.4 16.2 1 Explanation mol You may find additional useful data in the ALEKS Data tab. Does the following reaction absorb or release energy? (1) Co (g) Co (g) + e kJ mol kJ mòl Is it possible to calculate the amount of energy absorbed or released by reaction (1) using only the data above? kJ mol If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (1): Does the following reaction absorb or release energy? Co(g) + e Check Is it possible to calculate the amount of energy absorbed or released by reaction (2) using only the data above? If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (2): Q Search release O absorb O Can't be decided with the data given. O yes O no kJ/mol O release O absorb O Can't be decided with the data given. O…arrow_forwardWhat is the correct set of quantum numbers (n, I, ml) for the first electron removed in the formation of a cation for magnesium? 2,3,0 3,0,0 2,0,0 3,1,0 3,0,1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY