
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
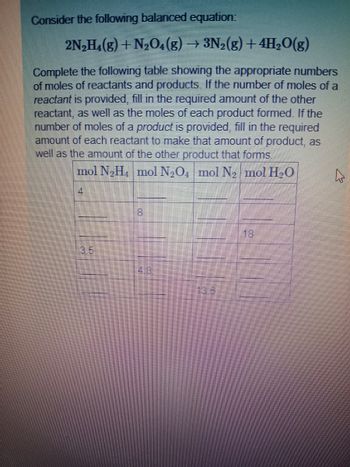
Transcribed Image Text:Consider the following balanced equation:
2N₂H₁(g) + N₂O4 (g) → 3N₂(g) +4H₂O(g)
Complete the following table showing the appropriate numbers
of moles of reactants and products. If the number of moles of a
reactant is provided, fill in the required amount of the other
reactant, as well as the moles of each product formed. If the
number of moles of a product is provided, fill in the required
amount of each reactant to make that amount of product, as
well as the amount of the other product that forms.
mol N₂H₁ mol N₂O4 mol N₂ mol H₂O
4
4

Transcribed Image Text:Search
Complete the following table showing the appropriate numbers
of moles of reactants and products. If the number of moles of a
reactant is provided, fill in the required amount of the other
reactant, as well as the moles of each product formed. If the
number of moles of a product is provided, fill in the required
amount of each reactant to make that amount of product, as
well as the amount of the other product that forms.
mol N₂H₂ mol N₂O, mol N₂ mol H₂0
4
35
8
48
13.6
18
D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step 1: Introduce to the concept of Stoichiometry
VIEW Step 2: Finding the stoichiometric relation
VIEW Step 3: Finding the mole of each species when 4 moles of hydrazine is taken
VIEW Step 4: Finding the mole of each species when 8 moles of dinitrogen tetra oxide is taken
VIEW Step 5: Finding the moles of each species when 18 moles of water has formed
VIEW Step 6: Finding the mole of each species when 3.5 moles of hydrazine is taken
VIEW Step 7: Finding the mole of each species when 4.8 moles of dinitrogen tetra oxide is taken
VIEW Step 8: Finding the moles of each species when 13.6 moles of nitrogen is formed
VIEW Solution
VIEW Step by stepSolved in 9 steps with 88 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How much (in gram) of HCl is required to produce 21.45 g of AlCl3 if the percentage yield for the following reaction is 61.24% ? Enter your answer without units. Molar masses (in g/mol):Al = 26.98H2 = 2.016HCl = 36.46AlCl3 = 133.34 2Al(s) + 6 HCl (aq) ⟶⟶ 2 AlCl3(s) + 3H2(g)arrow_forwardA 3.82-g sample of magnesium nitride is reacted with 7.73 g of water: Mg3N2 + 3H2O → 2NH3 + 3MgO The yield of MgO is 3.60 g. What is the percent yield in the reaction?arrow_forwardFor the reaction Ti(s) + 2 F2 (g) → TIF4(s) compute the theoretical yield of the product (in grams) for each of the following initial amounts of reactants.arrow_forward
- Calculate the theoretical yield of the product in moles for each of the initial quantities of reactants. Ti(s) + 2Cl2(g) -> TiCl4(s)arrow_forwardConsider the following reaction between sulfur trioxide and water: SO3(g)+H2O(l)→H2SO4(aq) A chemist allows 61.5 g of SO3SO3 and 11.2 g of H2O to react. When the reaction is finished, the chemist collects 56.2 g of H2SO4 Determine the percent yield for the reaction. Express your answer to three significant figures.arrow_forwardConsider the following balanced equation for the combustion of butane, a fuel often used in lighters.2 C4H10(g) + 13 O2(g) → 8 CO2(g) + 10 H2O(g)Complete the following table, showing the appropriate masses of reactants and products. If the mass of a reactant is provided, fill in the mass of other reactants required to completely react with the given mass, as well as the mass of each product formed. If the mass of a product is provided, fill in the required masses of each reactant to make that amount of product, as well as the mass of the other product that is formed. Mass C4H10 Mass O2 Mass CO2 Mass H2O _____ 2.11 g _____ _____ 5.32 g _____ _____ _____ _____ _____ 13.12 g _____ _____ _____ _____ 8.44 g 252 mg _____ _____ _____ _____ _____ 198 mg _____arrow_forward
- For the following reaction, 0.224 moles of sulfuric acid are mixed with 0.417 moles of calcium hydroxide. sulfuric acid (aq) + calcium hydroxide(s) → calcium sulfate(s) + water() What is the formula for the limiting reagent? Limiting reagent: What is the maximum amount of calcium sulfate that can be produced? Amount = molesarrow_forwardGIVEN C4H10 (g) + O2(g) → CO2 (g) + H2O (l) - Balance this reaction equation - Calculate the mass of CO2 produced when 10.5 g of C4H10 react with 15.0 g of O2. - What is the experimental yield if the percent yield in one experiment is found to be 75.5%?arrow_forwardFor the following reaction, 23.1 grams ofcarbon disulfide are allowed to react with89.2 grams of chlorine gas .carbon disulfide ( s ) + chlorine ( g ) carbon tetrachloride ( l ) + sulfur dichloride ( s )What is the maximum amount of carbon tetrachloride that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? gramsarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY