
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Consider the equilibrium between molecular iodine and atomic iodine in the gaseous state. For this endothermic reaction at 727°C, Kc = 3.8 x 10-5.
a. Are reactants or products favored at 727°C? How do you know?
b. Which way will the reaction shift (toward reactants or products?) to reach equilibrium if the reaction vessel (at 727°C) contains 1.0 M I2 (g) and 0.2 M I(g)? How do you know?
c. What is one change you could make to shift the reaction toward products? Why/how does that change work?
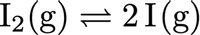
Transcribed Image Text:I2(g) = 21(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the reversible reaction: 22 2CO(g) + 2NO(g) = N2(g) + 2CO2(g) the following concentrations of reactants and products are found at equilibrium. What is the value of the equilibrium constant, K. under these conditions? [N2] = 1.57M, [CO2] = 0.668M, [CO] = 0.115M, [NO] = 0.227Marrow_forward#5 Q3arrow_forwardfollowing equilibrium reaction at 160.00 °C. If 0.420 atm of H2O2(g) is initially present, calculate the equilibrium partial pressures of H2(g), O2(g), and H2O2(g).arrow_forward
- The following reaction is product-favored at equilibrium at room temperature. C,H2 (g) + H2 (g) → C,H4 (g) Will raising or lowering the temperature make it reactant-favored? Raising the temperature can make it reactant-favored. O Lowering the temperature can make it reactant-favored. O Neither raising nor lowering the temperature can make it reactant-favored.arrow_forward6. Determine the equilibrium constant (Kp) for the following reaction at 25°C. a. 2H20 (l) —> 2H2 (g) + O2 (g) b. 2O3 (g) —> 3O2 (g) Thank you!arrow_forwardConsider the following equilibrium reaction: 2 NO (g) + O2 (g) ⇋⇋ 2 NO2 (g) What is the Kc expression? Select one: a. Kc=[NO2][NO][O2]Kc=[NO2][NO][O2] b. Kc=[NO2]2[NO]2+[O2]Kc=[NO2]2[NO]2+[O2] c. Kc=[NO]2[O2][NO2]2Kc=[NO]2[O2][NO2]2 d. Kc=2∗[NO2]2∗[NO][O2]Kc=2∗[NO2]2∗[NO][O2] e. Kc=[NO2]2[NO]2[O2]arrow_forward
- Consider the decomposition 4 PH3 (g) → P4 (s) + 6 H₂ (g) (Hrxn = -5.4 kJ/mol) Given the equilibrium constant for this process at 25°C is 233, what will be the value of the equilibrium constant at 37°C? (3 sf) Type your answer... of phosphene gas to form solid phosphorus and release hydrogen gas:arrow_forwardConsider the following endothermic reaction: 4 PCl5 (g) ⇋⇋ P4 (s) + 10 Cl2 (g) How will the amount of Cl2 be affected if the total pressure of the system is increased? Select one: a. The concentration of Cl2 will increase. b. The concentration of Cl2 will decrease. c. The amount of Cl2 will not change.arrow_forwardplease answer all parts!arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY