
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
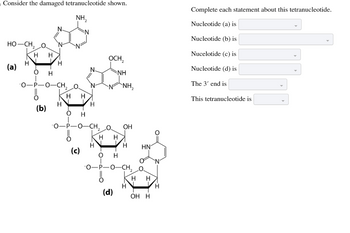
Transcribed Image Text:Consider the damaged tetranucleotide shown.
NH₂
HỌ—CH, O
(a)
H
HH
O
H
H
-O-P-O-CH₂ O
(b)
H
N
H H
(c)
H
OCH₂
O H
-O-P-O-CH₂ O
H
NH
N ANH,
NH₂
H H
OH
(d)
H HN
O H
-O-P-O-CH₂ O
H
H H
OH H
H
Complete each statement about this tetranucleotide.
Nucleotide (a) is
Nucleotide (b) is
Nucelotide (c) is
Nucleotide (d) is
The 3' end is
This tetranucleotide is
▶
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hello, I need help on my chemistry practice worksheet. I need help on number one (all the parts). Thank youarrow_forwardDelete atoms and charges, and add bonds as necessary to form a sphingomyelin. Select Draw Templates Groups More / / / C 0 N P H OH wwwwww. NH3 OH F R OH €arrow_forward17 Provide the abbreviation of the following nucleotide. ofotored H OH OH d A G C T U MP DP TP deoxyribose NH NH₂arrow_forward
- Consider the following DNA fragment. Identify the 5' and 3' ends, and label each of the nitrogenous basesarrow_forward12. Draw L-alanine as it appears at physiological pH. What is the absolute configuration of the alpha carbon? ( 13. Identify the polar and nonpolar parts of the assembly. Identify the pocket and the two bilayers that make up the assembly. Omon wo 0mm ww0 Ommnimo Omnimo Omnimo Omnimo Omm mo Omnimo Om ww Om ww Om ww Omm wwarrow_forwardName this tripeptide using 3 Potter abbreviations separated by hyphens (ie. G14-Pha-Play 艹 1 - 2 - 8 - - 7 - 8 -7 - $ 20 - GHz Off H₂C-CH citz ¹) Name 2) how many peotide tonds in the structure?arrow_forward
- 18. Based on the following nucleotide structure, answer the following questions: NH₂ R -OCH₂ a. OH OH Is the base present in this nucleotide a purine or a pyrimidine? b. What is the abbreviation for the base present in this nucleotide? (A, T, C, G, U) c. Would the sugar in this nucleotide be found in molecules of DNA or RNA or both? d. Would the base in this nucleotide be found in molecules of DNA, RNA or both? a baco:arrow_forwardquestion 28arrow_forwardDeoxyadenosine monophosphate (dAMP) and guanosine monophosphate (GMP) are nucleotides. The similarities between dAMP and GMP are that they both have? -an alpha (central) carbon.-the same R group.-a phosphate group.-a pentose (5 sided) sugar-an amino group-a nitrogenous base.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY