
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
( do D , E with explanation asap)
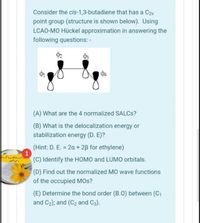
Transcribed Image Text:Consider the cis-1,3-butadiene that has a C2v
point group (structure is shown below). Using
LCAO-MO Hückel approximation in answering the
following questions: -
(A) What are the 4 normalized SALCS?
(B) What is the delocalization energy or
stabilization energy (D. E)?
(Hint: D. E. = 2a + 2B for ethylene)
(C) Identify the HOMO and LUMO orbitals.
(D) Find out the normalized MO wave functions
of the occupied MOs?
(E) Determine the bond order (B.0) between (C1
and C2); and (C2 and C3).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Aktiv Chemistry ← → C https://canvas.tamu... Jobs For Aggies - H... app.101edu.co 76°F Mostly cloudy Success Confirmation of Question X Draw the major product of this elimination. Consider regiochemistry and stereochemistry. Ignore byproducts. Br H₂O Jobs - Zoo Jobs Ne... heat + Internships | Job Ca... internship paper Your Online Accoun... Question 6 of 21 99+ Urban Wildlife Infor... A Site Suitability An... Please select a drawing or reagent from the question area Aktiv Learning Submit X 10/31/2022 : 4:06 PM sarrow_forwardHELP ASAP! please write out mechanism and reagants/reactants for thisarrow_forwardranslate Bb Welcome, Kawtha... O Maps GE News Home [Review Topics] [References) Draw the most stable resonance form for the intermediate in the following electrophilic substitution reaction. OH HO Br2 Br You do not have to consider stereochemistry. Include all valence lone pairs in youranswer. In cases where there is more than ore answer, just draw one. opy aste [* Previous Next Show Hint Email Instructor Save and Exit Cengage Learning | Cengage Technical Supportarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY