
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Use part A to solve part B of 20. Please write the answer clearly
![**Chemistry Exercise: Neutralization Reaction**
Consider the chemical reaction that takes place between aqueous hydrochloric acid and aqueous sodium hydroxide.
**Task:**
Complete the balanced neutralization equation for the reaction below:
\[ \text{HCl(aq) + NaOH(aq) → NaCl(aq) + H}_2\text{O(l)} \]
**Response:**
*Correct!*
**Correct Answer:**
\[ \text{HCl(aq) + NaOH(aq) → NaCl(aq) + H}_2\text{O(l)} \]
This exercise demonstrates a common neutralization reaction where an acid reacts with a base to produce a salt and water.](https://content.bartleby.com/qna-images/question/ed3baa28-3498-4406-bfcd-63c431614c83/89e8af2d-b240-4a38-a789-a7bcada08843/itbqdej_thumbnail.png)
Transcribed Image Text:**Chemistry Exercise: Neutralization Reaction**
Consider the chemical reaction that takes place between aqueous hydrochloric acid and aqueous sodium hydroxide.
**Task:**
Complete the balanced neutralization equation for the reaction below:
\[ \text{HCl(aq) + NaOH(aq) → NaCl(aq) + H}_2\text{O(l)} \]
**Response:**
*Correct!*
**Correct Answer:**
\[ \text{HCl(aq) + NaOH(aq) → NaCl(aq) + H}_2\text{O(l)} \]
This exercise demonstrates a common neutralization reaction where an acid reacts with a base to produce a salt and water.
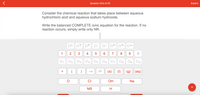
Transcribed Image Text:**Chemical Reaction: Hydrochloric Acid and Sodium Hydroxide**
Consider the chemical reaction that takes place between aqueous hydrochloric acid (HCl) and aqueous sodium hydroxide (NaOH).
**Task:**
Write the balanced complete ionic equation for this reaction. If no reaction occurs, simply write "NR."
**Ionic Equation Options:**
- Use the provided elements and charges to construct your equation:
- Elements: O, Cl, OH, Na, H
- Charges: \(0^-\), \(1^-\), \(2^-\), \(3^-\), \(4^-\), \(0^+\), \(1^+\), \(2^+\), \(3^+\), \(4^+\)
- States of matter: solid (s), liquid (l), gas (g), aqueous (aq)
- Symbols: plus (+), arrow (→)
**Notes:**
To solve this, consider the disassociation of HCl and NaOH in water and combine to form water (H₂O) and sodium chloride (NaCl). Write these reactions using charged ions:
- \( \text{H}^+ (aq) + \text{Cl}^- (aq) + \text{Na}^+ (aq) + \text{OH}^- (aq) \rightarrow \text{H}_2\text{O} (l) + \text{Na}^+ (aq) + \text{Cl}^- (aq) \)
Cancel spectator ions (Na⁺ and Cl⁻) to form the net ionic equation:
- \( \text{H}^+ (aq) + \text{OH}^- (aq) \rightarrow \text{H}_2\text{O} (l) \)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- ||| cloudy CHEMICAL REACTIONS Writing net ionic equations The following chemical reaction takes place in aqueous solution: SnSO₂(aq)+2 NaOH(aq) → Sn(OH)₂(s)+Na₂SO4(aq) Write the net ionic equation for this reaction. 10 Explanation Check Search L X 00 S 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privac =arrow_forwardI don’t get it at allarrow_forwardA solution of HCl at a certain temperature has a density of 1.189 g/mL. The concentration of the solution is © Time left: 96:06 Begin 12.39M automatic instructions Chores Additional data: Students Molar mass of HCl - 36.458 q/mol Questions Molar mass of H20 - 18.016 g/mol Ask My classes Question 2 Given or above calculate:arrow_forward
- 10 Homework • Unanswered CO2(8) HCl(aq) + _Na2C03(aq) →_NaCl(aq)+_CO2(e) + _H2O(1) Identify the net ionic equation. Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. 2H*(aq)+ CO (aq)→CO2(g)+H2O(1) a b H*(aq)+ CO-(aq)-CO2(g)- H20(1I) 2Na+ (aq)+2CI¯(aq)→2NaCl(s) d 2H+ (aq)+ 2Cl-(aq)→2HCI(s)arrow_forwardSuppose i want to manufacture concentrated bubble liquid for bubble guns. Now i want the master formula or working formula how to make this concentrated bubble liquid? What are the ingredients along with concentration ans step by step procedure how to make it? I want to make 10 ml concentrated bubble liquid for this please give a master formula? I will rate you positive if you do this. Please don't use AI for answering this question.arrow_forwardWhat are the products for the net ionic reaction that would occur when an aqueous solution of Li2CO3 is combined with an aqueous solution of Ca(OH)2? [Select all that apply.] Group of answer choices Ca2+ (aq) CaCO3 (s) OH- (aq) Li+ (aq) Ca2CO3 (s) CO32- (aq) LiOH (s) H2O (l)arrow_forward
- ANALYSIS PAGE Click on the number to put it in your LabPad Calculator H₂SO, volume: 25 mL H₂SO4 diluted to: 10% Volume of NaOH : 0.03039 L NaOH mol in volume : 0.00456 mol BASE to ACID : 2:1 H₂SO, moles : 0.00228 mol m RESULTS TABLE 2 NaOH + 1 H₂SO, → 2 H₂O + 1 Na,So, ww: X ENTER TOTAL 2arrow_forwardTools Window Help View History Bookmarks ... 71% D Fri 3:25 PM A writing.citationmachine.net Editor 101 Chem101 Calculate the volume of CO, ga: X + e Share -> 8 https://app.101edu.co ate Sensitivit Question 13 of 13 Submit Gra Plag When excess water is added to a mixture of citric acid and sodium Expe bicarbonate present in a bath bomb, the following reaction takes place C6H&O7(s) + 3 NaHCO3(s) + H2O(1) → Na3C6H;07(aq) + 4 H20(1) + 3 CO2(g) Calculate the volume of CO2 gas collected over water at 38.0 °C when a mixture of 55.5 g of citric acid and excess sodium bicarbonate is added to a tub of water if the total pressure is 725 torr. The vapor pressure of water at 38.0 °C is 49.7 torr. L 4 6 C 7 8 +/- x 100 O Focus General 书 1263 words English (United States) 2450 word: Page 2 of 5 Page 1 of 7 Page 1 of 3 816 words tv NOV 19 MacBook Air DII F10 F9 80 888 FB F7 F6 F5 F4 esc F2 F3 | & 3. 1,arrow_forwardTHIS IS WRONG PLEASE HELP BY GIVING RIGHT ANSWER. THIS IS A CHEMISTRY PROBLEM, NOT A WRITING OR ESSAY ASSIGNMENT!!!!!!!!arrow_forward
- just give answers please and label answer so I knowarrow_forwardShow work One ingredient in some antacids is Al(OH)3 which reacts with stomach acid, HCl . Complete and balance the equation for this neutralization reaction written below. __?__Al(OH)3 + __?_ HCl → __?____ + __?____arrow_forwardWhen the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: Cl2 (g) + H20 (1) – HCI (aq) + |HCIO3 (aq) Submit Answer Try Another Version 9 item attempts remainingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY