
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Bb Chem101 (Homework & Class As X
Chem101
G Consider the balanced chemical
A app.101edu.co
Question 1 of 11
Submit
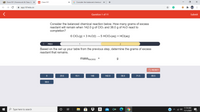
Consider the balanced chemical reaction below. How many grams of excess
reactant will remain when 142.0 g of CIO2 and 38.0 g of H2O react to
completion?
6 CIO-(g) + 3 Н-0() — 5 НСIO:(aq) + HCІ(аq)
PREV
1
2
Based on the set up your table from the previous step, determine the grams of excess
reactant that remains.
massexcess
%3D
5 RESET
29.6
19.1
148
142.0
38.0
71.5
89.5
38.6
11:54 AM
P Type here to search
a
11/1/2020
近
Transcribed Image Text:Bb Chem101 (Homework & Class As X
Chem101
G Consider the balanced chemical
A app.101edu.co
Question 1 of 11
Submit
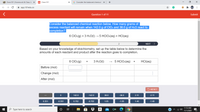
Consider the balanced chemical reaction below. How many grams of
excess reactant will remain when 142.0 g of CIO2 and 38.0 g of H2O react to
completion?
6 CIO:(g) + 3 H20(1) → 5 HCIO:(aq) + HCI(aq)
1
NEXT
>
Based on your knowledge of stoichiometry, set up the table below to determine the
amounts of each reactant and product after the reaction goes to completion.
6 CIO:(g)
3 H2O(1)
5 HCIO:(aq)
HCI(aq)
Before (mol)
Change (mol)
After (mol)
5 RESET
142.0
-142.0
38.0
-38.0
2.11
-2.11
0.351
-0.351
0.702
-0.702
1.05
-1.05
1.40
-1.40
11:53 AM
P Type here to search
a
11/1/2020
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- SS a. For the following balanced chemical equation, calculate how many moles of products would be produced if 0.255 mol of the first reactant were to react completely. CO₂(g) + 4H₂(g) → CH4 (g) + 2H₂O (1) mol CH₂ mol H₂O b. For the following balanced chemical equation, calculate how many moles of products would be produced if 0.790 mol of the first reactant were to react completely. BaCl₂ (aq) + 2AgNO3(aq) → 2AgCl(s) + Ba(NO3)2 (aq) mol AgCl mol Ba(NO3)2 c. For the following balanced chemical equation, calculate how many moles of products would be produced if 0.815 mol of the first reactant were to react completely. C3H8 (9)+502(g) → 4H₂O(1) + 3CO2(g) mol H₂O mol CO2 Submit Answer Retry Entire Group Show Hint 9 more group attempts remaining 19 Previous Nextarrow_forward[References] TUTOR Chemical Equations: Gram/Gram Calculation Consider the reaction: SOe) + H;O(1) → H3SO;( Given an initial mass of 15.3 g SO, an excess of H50, and assuming that all of the reactant is converted to product(s), and none is lost, calculate the mass (g) of H7SO3 produced by the reaction.arrow_forwardThe reaction of boron and chlorine can be expressed as the following chemical equation: 2 B + 3 Cl2 ==> 2 BCl3 If 47.2 grams of boron completely reacts with 121.2 grams of chlorine gas, then how many grams of the EXCESS REACTANT will be leftover?arrow_forward
- For the reaction shown, calculate how many moles of each product form when the given amount of each reactant completely reacts. Assume that there is more than enough of the other reactant.C3H8(g)+5O2(g)→3CO2(g)+4H2O(g) 0.0500 molO2 to mol CO2 Express your answer using three significant figures.arrow_forwardA sample of 12.3 g of Fe₂O3 reacts with 11.1 g CO to yield Fe and CO₂. The balanced chemical equation is Fe₂O3(s) + 3 CO(g) - ► 2 Fe(s) + 3 CO₂(g) Which substance is the limiting reactant? O Fe₂O3 CO Fe CO₂ What is the theoretical yield of Fe? mass of Fe: If the actual experimental yield for Fe is 7.92 g, what is the percent yield of Fe? garrow_forwardGiven the (fictional) balanced equation: 2 Q(s) + 5 G (g) → Q2G5 (s) And the following formula masses: Compound: Formula mass (g/mol) 1.00 G. 2.00 Q2G5 12.00 Determine how many moles of G are required to fully react with 43 moles of Q to produce Q2G5.arrow_forward
- Ammonium nitrate has been used as a high explosive because it is unstable and decomposes into several gaseous substances. The rapid expansion of the gaseous substances produces the explosive force. NH,NO3 (8) → N2 (g)+ O2 (g) + H2O(g) Calculate the mass of each product gas if 47.4 g of ammonium nitrate reacts. g N2 g O2 g H2Oarrow_forward10.arrow_forwardAl2Cl6 is made by treating scrap metal with chlorine gas according to the following equation: 2 Al (s) + 3 Cl2 (g) ⟶⟶ Al2Cl6 (s) In the reaction of 45.9 g Al with 212.5 g Cl2, determine the limiting reactant and theoretical yield of the product. The limiting reactant is? The theoretical yield of Al2Cl6 is?. Round the answer to 1 decimal place. If the reaction produces 139.4 g Al2Cl6, calculate the percent yield of the reaction. ? %. Round the answer to 1 decimal place.arrow_forward
- When aluminum oxide reacts with sulfuric acid, aluminum sulfate and water are produced. The balanced equation for this reaction is: Al2O3 (s) + 3H2SO4 (aq) → Al2(SO4)3(aq) + 3H₂O(1) [Review Topics] [References] Use the References to access important values if needed for this question. If 12 moles of sulfuric acid react, The reaction consumes The reaction produces Submit Answer moles of aluminum oxide. moles of aluminum sulfate and moles of water. Retry Entire Group 2 more group attempts remaining Cengage Learning | Cengage Technical Support Previous Email Instructor Next Save and Exiarrow_forwardSulfur dioxide gas reacts with sodium hydroxide to form sodium sulfite and water. The unbalanced chemical equation for this reaction is given below: SO2(g) + NaOH(s) → Na2SO3(s) + H2O(l) Assuming that you start with 55.3 g of sulfur dioxide and 32.3 g of sodium hydroxide and assuming that the reaction goes to completion, determine the mass of each product formed. -----gNa2SO3 -----gH2Oarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY