
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Please solve using the formula sheet if needed

Transcribed Image Text:Plug flow reactor
Problem 1
Consider a reaction A⇒B in a liquid phase with a second order reaction, kÃ=0.1 L/mol/hr. The feed
contains 2 mol/L of A and enters the reactor with a flowrate of 1 mol/hr. Calculate the volume of a plug
flow reactor to reach 75% conversion.
Repeat the same calculation for first order and zero order reactions.
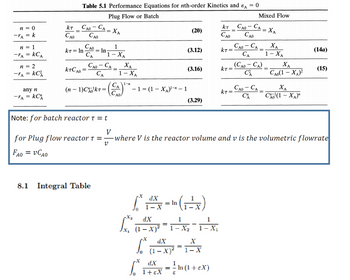
Transcribed Image Text:n = 0
-TA = k
n = 1
-TA = KCA
n = 2
-TA = KC²
any n
=
-TA
kC
KT
CAO
Table 5.1 Performance Equations for nth-order Kinetics and A = 0
Plug Flow or Batch
CAO - CA
CAO
KT = ln
CAO
CA
kTCao =
= In
CAO
CA
(n − 1)Chokr=
8.1 Integral Table
= XA
1
1-XA
- CA
ΧΑ
1-XA
C₁ 1-n
A0/
- 1 = (1 - XA)¹-n – 1
pX₂
(20)
(3.12)
dX
(1
(3.16)
dX
(4=(x)
In
1- X
(3.29)
1- X
x₁²01²²x²-1-²-²₂2-1-²X
dX
fax-x
X
1- X
KT
CAO
dX
²14xx - ² m (1 + ex)
= ln
+ EX
KkT=
KT=
KT =
САО - СА
CAO
CAO - CA
CA
Note: for batch reactor t = t
V
for Plug flow reactor t = where V is the reactor volume and v is the volumetric flowrate
V
FAo = vCAO
Mixed Flow
(Cao – CA)
-
ÁO - CA
CA
= XA
XA
1 - XA
XA
CA0(1-XA)²
ΧΑ
Cho (1 − XA)"
(14a)
(15)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 9 images

Knowledge Booster
Similar questions
- . (Grinding Operations and Grinding Machines) (USCS units) In a centerless grinding operation, the grinding wheel diameter = 8 in, and the regulating wheel diameter = 5.0 in. The grinding wheel rotates at 1500 rev/min, and the regulating wheel rotates at 180 rev/min. The inclination angle of the regulating wheel = 2.3°. Determine the production rate of cylindrical work parts whose diameter = 0.5 in and length = 5.0 in. Solutions used; fr = TD₂N, sin Iarrow_forwardThis is a picture of a 10-mL graduated cylinder. The value for 'x' says 3 mL and the value for 'y' says a value 1 mL higher than 3. Record the value with proper estimation technique.arrow_forwardWhat do you mean by volumetric flow rate?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The