
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
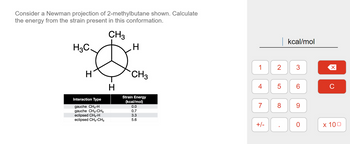
Transcribed Image Text:Consider a Newman projection of 2-methylbutane shown. Calculate
the energy from the strain present in this conformation.
CH3
H3C.
H
Interaction Type
gauche CH3-H
gauche CH3-CH3
eclipsed CH3-H
eclipsed CH3-CH3
H
H
CH3
Strain Energy
(kcal/mol)
0.0
0.7
3.3
5.6
1
4
2
+/-
LO
5
7 8
kcal/mol
3
CO
6
9
O
X
C
x 100
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the energy diagram showing the conformational analysis of propane and match the indicated position with the correct conformation below. 00000 - = 3 2 OIV OV Н. CH3 Н Н H пи m 0 dihedral angle energy 180 HH H Н II Н. H Н Ш CH3 H 180 HCH3 HXH Н IV HCH3 CH3 HXH Н Varrow_forwardreq Zreq 2req 2req 2req [Review Topics) [References] Cyclohexane derivatives exist primarily in the most stable of the available chair conformations. Give the position, axial or equatorial, of each of the three groups shown when the ring is in the most stable chair conformation. If a group divides its time equally between axial and equatorial positions, indicate this with ax/eq. The table of "Axial Strain Energies for Monosubstituted Cyclohexanes" found in the "Strain Energy Increments" section of the Reference tool is useful for answering this question. OH HC Group a is Group bis Group cis Submit Answer OH b Retry Entire Group 9 more group attempts remaining o O · 9020 0arrow_forwardUsing your model kits, make trans-1-tert-butyl-3-methylcyclohexane. Draw the least stable and most stable chair conformation. The structures must be related as chair inversions. Least stable Chair Conformation Most stable Chair Conformation Sl bom on chiraarrow_forward
- 2. Our knowledge of chair conformations allows us to use them to determine a great deal about a reaction's mechanism. In particular, using a t-butyl group to 'fix' a cyclohexane in a single conformation can help us learn about the spatial requirements and energetics of a reaction. Consider the following two reaction products: a. NO NID ALOH 1) NaCN 2) H+ 1) NaBH4 2) H3O+ OH Group -H -CN -OH A Value (kcal/mol) Defined as 0 0.2 kcal/mol 1.0 kcal/mol Draw the most stable chair conformation for each of the two products. b. Based on the A values in the table above, do the two products have the most stable conformation at the new stereocenter?arrow_forwardCalculate strain energy for the conformed pictured below, using strain energy increments from the table.arrow_forwardComplete the question on paper and snap a photo and upload it as your submission in jpg or pdf format. CI IO H OH a. Generate a Newman projection of the molecule shown above. The red arrow indicated the direction from which your eyes are looking at the molecule. b. Also draw a Newman projection of a conformation that is less stable than the one that you have draw in a. There may be more than one correct option to generate a less stable Newman projection - you need only generate one. You can rotate around the C-C single bond to go between eclipsed and/or staggered conformations. c. Describe why the conformation in b. is less stable than a (name the interactions that make the conformation less stable). Point out where on your structure those interactions are (circling is fine).arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY