
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Three students did a chromatography experiment, where Rf = distance of solute / distance of solvent.
What could be the possible errors why student 3 had results that are quite far from that of students A and B?
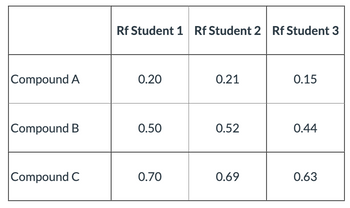
Transcribed Image Text:Compound A
Compound B
Compound C
Rf Student 1 Rf Student 2 Rf Student 3
0.20
0.21
0.15
0.50
0.52
0.44
0.70
0.69
0.63
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- [1] In paper chromatography, why do some ions move only a short distance from the point of application while others move much more?arrow_forwardA student performed chromatography on a mixture of benzoic acid (C6H5CO₂H), acetophenone (C6H5C(O)CH3) and styrene (C6H5CHCH₂). She used alumina (Al₂O3) as the stationary phase and a 9 : 1 mixture of hexane : ethyl ethanoate as the mobile phase. Rank the order in which the compounds moved, from fastest to slowest. benzoic acid acetophenone styrenearrow_forward5arrow_forward
- A2arrow_forwardA young researcher was evaluating a standard method for determining the methylmercury content in blue fin tuna using high performance liquid chromatography (HPLC). She determined the standard deviation (?)(s) for the method to be 0.440.44 ppb and assumed that ?s was a good approximation of ?σ . As a test, she used this method to evaluate the methylmercury content in a National Institutes of Standards and Technology (NIST) standard and determined the unknown amount to be within 0.210.21 ppb (?)(μ) of the known mean with 99% probability. How many replicate measurements of the NIST standard did the researcher perform?arrow_forward6. The concentration of caffeine in an energy soft drink was determined by standard addition using HPLC. The following procedure was used: a standard caffeine solution was prepared by adding 0.09850g of caffeine to 25.0 cm3 of water.A series of solutions were prepared by adding known volumes of the standard solution to the soft drink before diluting to 100 cm3. The volume of standard and the caffeine peak area are shown in the table: Energy Drink (cm3) Added caffeine Std (cm3) Peak area 15 0 965 15 5 1352 15 10 1739 15 15 2126 15 20 2513 Determine the concentration of caffeine in the soft drink as mg cm-3. A. 3.3 B. 2.3 C. 4.3 D. 7.3arrow_forward
- In chromatography, it is desirable to increase the resolution. a) increase the plate height (H) b) decrease the plate height (H) c) decrease the number of plates (N) toarrow_forward-3 A 0.3202 (±0.0001) g sample of a pure monoprotic acid required 20.04 (±0.02) cm° of 0.1005 (±0.0005) mol dm neutralisation. Find the molar mass of the acid in g mol together with its uncertainty also in g mol, with an appropriate number of digits. NaOH for -1 Select one: a. 1.59 x 102+ 0.2 g mol b. 1.60 x 10 + 0.03 g mol C. 1.590 x 102 + 0.03 g mol d. 1.590 x 10- + 0.2 g mol 1.60 x 10-+0.2 g mol е. Clear my choicearrow_forwardWhich statement(s) are correct for gas chromatography? A) the components in a mixture can be identified from their retention time. B) the relative peak areas give the proportions of components in a mixture. C) Calibration curves are used to confirms the concentrations of components in a mixture.arrow_forward
- In a particular trial, a student got a value for R of 0.083. Calculate the percent deviation of this result. Enter a number without the % sign. The percent deviation should be reported as a positive value.arrow_forward4.Consider the peaks for pentafluorobenzene and benzene in the gas chromatogram shown here. The elution time for unretained solute is 1.06 min. The open tubular column is 30.0 m in length and 0.530 mm in diameter, with a layer of stationary phase 3.0 μm thick on the inner wall. a) Measruing the width, w, at the baseline on the chromatogram, find the number of plates for these two compounds b) Use your answer to (a) to find the resolution between the two peaks c) Using the number of plates N=sqrtN1*N2 with the values from (a) calcuate what the resolution should be and compare your answer with the measured resolution in barrow_forwardA quality-control laboratory analyzes a product mixture us-ing gas-liquid chromatography. The separation of components ismore than adequate, but the process takes too long. Suggest twoways, other than changing the stationary phase, to shorten theanalysis timearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY