
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
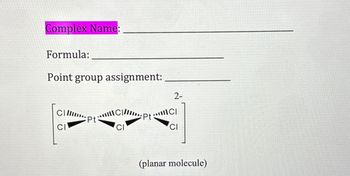
Transcribed Image Text:Complex Name:
Formula:
Point group assignment:
CIII
CI
C|II
...ptC/
Pt
2-
CI
CI
(planar molecule)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- anic chemistry II 4) Which of the following compounds can exhibit fac-mer isomerism? a) [Ir(NH3)4Br₂]* b) [Ru(CO),ONO]²+ II: [Pt(dien)CI]* c) [Mo(CO), (NH3)3] ³+ 5) What is the symmetry point group for (cis-NiF₂Br₂²) (b) C₂v (a) D₂h (c) C₂h 6) Which of the following complexes are chiral? I: [Ru(en)3]² III: a) Only III 7) Which of the following will give a white precipitate upon reacting with AgNO3? c) [Cr(H₂O),]Cl; b) [Ir(NH3)3Cl3] a) K₂[Ni(en)₂Cl₂] b) II and IV (d) Dah cis-[Cr(C₂O4)₂Cl₂]³- c) I and III d) [Ag(CO),F]* (e) Cs IV: trans-[Cr(C₂O4)2Cl₂]³¹ d) II and III d) [Fe(H₂O),Cl3]arrow_forwardThe complex Mo(CO)3(NCC2H5)3 has the infrared spectrum shown below. Is this complex more likely the fac or mer isomer? Explain demonstrating stretching vibrations?arrow_forwardEnter the steric number (SN) and coordination geometry (CG) of the central atom for each substancearrow_forward
- 1. Using the MO scheme provided below, calculate the bond order for the ligand-metal bonds in a ML6 complex where M=Co2+ and NH3: Recall that independent of the ligand, the eg orbitals that are 10Dq above the t2g orbitals are always o*. M ML6 6L O* 4p d,?.? d,? 2 х - у 4s 3d dy dyz dyz sp3 Op Osarrow_forwardwhat's the point group of an octahedron with one blue face, the others yellowarrow_forwardWhy Cs2[Cu(CN)4] doesn't exhibit a Jahn-Teller distortionarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY