
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
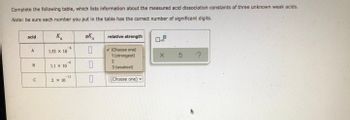
Transcribed Image Text:Complete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids.
Note: be sure each number you put in the table has the correct number of significant digits.
acid
K₁
pK
relative strength
x10
A
0
✓ (Choose one)
1.53 x 10
1 (strongest)
3 ?
2
0
1.1 X 10
3 (weakest)
0
(Choose one)
2. X 10
B
00
C
-6
-6
-11
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids. Note: be sure each number you put in the table has the correct number of significant digits. Ka pk a acid relative strength x10 A 2.36 x 10 (Choose one) В 7.0 (Choose one) C 3.50 (Choose one)arrow_forwardComplete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids. Note: be sure each number you put in the table has the correct number of significant digits. K. relative strength acid x10 |(Choose one) olo A 3.08 -11 В 2.01 X 10 (Choose one) Ar C 6.0 v (Choose one) 1 (strongest) 3 (weakest)arrow_forwardWhich acid ionization constant would indicate the weakest acid? Group of answer choices 3.5 x 10–4 1.8 x 10–5 4.6 x 10–4 1.5 x 10–2arrow_forward
- Acids & Bases: Understanding Connections Between Descriptions of Weak Acid Dissociation In an aqueous solution of a certain acid the acid is 0.054% dissociated and the pH is 4.49. Calculate the acid dissociation constant Ka of the acid. Round your answer to 2 significant digits.arrow_forwardComplete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids. Note: be sure each number you put in the table has the correct number of significant digits. pka acid A B с Ka 2. × 10 7.38 x 10 10 11 4.11 relative strength (Choose one) (Choose one) (Choose one) x10 Xarrow_forwardComplete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids. Note: be sure each number you put in the table has the correct number of significant digits. Ka pK a acid relative strength x10 A ✓ (Choose one) 1. X 10 X 5 ? 1 (strongest) B 0 2 3 (weakest) C (Choose one) 8.7 X 10 1 7.022 0arrow_forward
- Please use the values in the resources listed below instead of the textbook values. Which is the stronger acid, C5H5NH+ or HCNO? (See the Acid and Base Dissociation Constants table. They are considered of equal strength if they are within 5% of each other. Assume Kw = 1.01x 10-14.) C5H5NH + HCNO They are of equal strength.arrow_forwardComplete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids. Note: be sure each number you put in the table has the correct number of significant digits. K. pka acid relative strength x10 v (Choose one) 1 (strongest) A 6.021 2 В 7.30 3 (weakest) 6- C 1. X 10 (Choose one) ♥arrow_forwardComplete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids. Note: be sure each number you put in the table has the correct number of significant digits. K. pKa acid relative strength x10 1.315 (Choose one) alo А В 0.9 (Choose one) Ar C 1.3 х 10 v (Choose one) 1 (strongest) 2 3 (weakest)arrow_forward
- The pH of a 1.3 M solution of hexanoic acid (HC ,H,02) is measured to be 2.37. Calculate the acid dissociation constant K, of hexanoic acid. Be sure your answer has the correct number of significant digits. K aarrow_forwardComplete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids. Note: be sure each number you put in the table has the correct number of significant digits. Ka pK. relative strength acid x10 А 5.79 (Choose one) e 4 В 2. X 10 (Choose one) C 11.764 (Choose one)arrow_forwardWhat are some sources of error when determining the Ka of an unknown weak acid.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY