
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
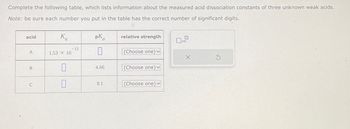
Transcribed Image Text:Complete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids.
Note: be sure each number you put in the table has the correct number of significant digits.
acid
A
B
C
K
1.53 × 10
0
0
12
pK
4.66
9.1
relative strength
(Choose one) ✓
(Choose one)
(Choose one) ✓
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The pH of a 1.3 M solution of hexanoic acid (HC ,H,02) is measured to be 2.37. Calculate the acid dissociation constant K, of hexanoic acid. Be sure your answer has the correct number of significant digits. K aarrow_forwardThe pH of a 1.2M solution of hexanoic acid (HC H,,0,) is measured to be 2.39. Calculate the acid dissociation constant K, of hexanoic acid. Be sure your answer has the correct number of significant digits. K a x10arrow_forwardFind the H3O+H3O+ concentration of a 0.220 MM hypochlorous acid solution (whose acid dissociation constant is Ka=2.9×10−8Ka=2.9×10−8). Express the concentration to two significant figures.arrow_forward
- The compound trimethylamine, (CH3)3N, is a weak base when dissolved in water. Write the K₁ expression for the weak base equilibrium that occurs in an aqueous solution of trimethylamine: Kb =arrow_forwardMany important biochemicals are organic acids, such as pyruvic acid (pka = 2.5) and lactic acid (pka = 3.86). For each acid, draw a simple titeation curve, and label the pKa. Draw an arrow where the line crosses pH 3 for each acid? Calculate the percentage of acid and conjugate base for both at pH 3..arrow_forwardComplete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids. Note: be sure each number you put in the table has the correct number of significant digits. acid B C Ka M 0 7.28 x 10 pK 1.17 11.3 0 relative strength (Choose one) ✓ (Choose one) ✓ (Choose one) ✓ O *10 X ?arrow_forward
- Complete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids. Note: be sure each number you put in the table has the correct number of significant digits. K. pk a acid relative strength x10 А 9.036 (Choose one) ? 6- 2.6 x 10 (Choose one) - 12 4. X 10 C (Choose one) B.arrow_forwardThe acid ionization constant, Ka, of a certain weak acid HA equals 1.4 x 10-11. What does Kb for the conjugate base, A- equal? Assume that the temperature equals 25 ˚C.arrow_forwardQ)1arrow_forward
- Complete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids. Note: be sure each number you put in the table has the correct number of significant digits. acid K pK relative strength x10 A ✓ (Choose one) X S ? 1 (strongest) B 2 3 (weakest) с (Choose one) Ba -5 8. × 10 5.89 × 10 -9 7.02 1 0arrow_forwardComplete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids. Note: be sure each number you put in the table has the correct number of significant digits. acid K pK relative strength x10 -8 A 4.24 x 10 ☐ B ☐ 1.46 C 5.9arrow_forwardIdentify the conjugation acid-base pairs in the following reactions. An acid donates a proton to become a conjugate base. A base accepts a proton to form a conjugate base.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY