
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
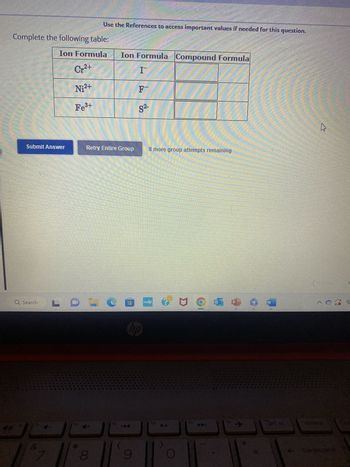
Transcribed Image Text:HIER
Complete the following table:
Ion Formula
Cr²+
Ni²+
Fe³+
WAR
430
Submit Answer
Q Search
JO
Use the References to access important values if needed for this question.
Retry Entire Group
+
8
fg
Ion Formula Compound Formula
I
F
S²-
hp
144
0050
DE
(10
LO-COPG MOJE OO
▶11
M
m
8 more group attempts remaining
HOR
CAN
f11
TER
112.
prt sc
←
^ G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- CrCl3CrCl3 Express your answer as an ion.arrow_forward=16834872&OpenVellumHMAC=765a9e0c4c56ffa4c558304d6cf2... Q ☆ eedback 30 of 33 Review I Constants I Periodic Table the Part A CH3-CH2–NH, + H2O = Draw the molecules on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars, including charges where needed. The single bond is active by default. ®, H: con H. H2 C.arrow_forwardent/takeCXPCompliantActivity.do?locator=Dassignment-take&takeAssignmentSessionLocator=assignment-take [References] MULATION lonic Compound Formulas Cations Anions O NH4 O Li CF O Br Na O Ca2+ Ba2+ O Ag O Fe2+ O Fe+ O AB+ O Pb2+ Name: ammonium fluoride OH O s- O co,? O so PO3- O NO3 O C104 Formula: NH,F 2- Solubility: soluble in water 2- Now examine the salts of Na with the anions that have -2 charges: S2, CO32, and SO How many Na cations are combined with each -2 anion in the compound formulas? (6 of 9) Rechedk 1st attempt Incorrect Incorrect FLVarrow_forward
- Fill in formula, namearrow_forwardWrite the chemical formula for ammonium phosphide |3+ 4+ 3 2 2+ 1 2 3 5 6 7 8 9 0 1 3 5 7 (aq) (s) (1) (g) PO3 ld NH3 P PO4 NH4 Ph Z LOarrow_forwardActivity Stre X app.101edu.co Tube Maps The Univers X Content Aktiv Chem X Chapter 13. X TGH Become a V X Question 14 of 19 Provide the correct systematic name for the compound shown here. O 80 F1 F2 F3 # $ ABBI 2 3 Q WE 69 4 R F4 X % 5 N- 2- F5 T 5- 4- 4- || N,N- | 3- di tetra tri prop meth but eth hex pent amine al amide MacBook Air F6 O) 6 Y ZI H & 7 F7 U J 00 * 8 Adult Volun X (97) Pintere X d Page no □ ☆ = DII F8 8 F9 9 D K 0 0 L 7 F10 P F11 + 11 [arrow_forward
- please answer quicklyarrow_forward* OWLV2 | Online teaching and lea x C For The Following Reaction, 6.15 x cengagenow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take [References] 1 pt Use the References to access important values if needed for this question. D 1 pt For the following reaction, 26.7 grams of carbon disulfide are allowed to react with 104 grams of chlorine gas. 1 pt CS2(s) + 4 Cl2(g) CC14(1) + 2 SCI2(s) > 1 pt 1 pt What is the FORMULA for the limiting reagent? 1 pt What is the maximum mass of carbon tetrachloride that can be formed? grams 1 pt What mass of the excess reagent remains after the reaction is complete? grams 1 pt 1 pt Submit Answer Try Another Version 1 item attempt remaining 1 pt 1 pt 1 pt 1 pt O 1 pt 1 pt D 1 pt Chparrow_forwardOWLV2 Online teachin X Cthe Deep Blue Compound ( + https://cvg.cengagenow.com/ilm/takeAssignment/takeCovalentActivity.do?locator%=Dassignment-take&takeAssignmentS [References] The deep blue compound Cu(NH3)4SO4 is made by the reaction of copper(II) sulfate and ammonia. CUSO4 (aq) +4 NH, (aq) → Cu(NH3)4SO4 (aq) If you use 27.0 g of CuSO4 and excess NH3, what is the theoretical yield of Cu(NH3),SO, ? g Cu(NH3)4SO4 If you isolate 22.0 g of Cu(NH3)4SO4, what is the percent yield of Cu(NH3)4SO4? Submit Answer Try Another Version 8 item attempts remaining Show Hint searcharrow_forward
- IMG_9522.HEIC Open with Preview lete the following table: Cation Formula Anion Formula Compound Formula Cr+ NO3 Cr(NO,)3 Cu²+ 02- Co2+ PO, 3-arrow_forwardTopic: Chapter 2 Materials and OWLV2 | Online teaching and lea X New tab Ô https://east.cengagenow.com/ilrn/takeAssignment/takeCXPCompliantActivity.do?locator=Dassignment-take CHAPTER 2 - ATOMS, MOLECULES, AND IONS OPrevious Page 1 of 2 Next O You are given 0.10 g samples of Li, Ca, Cr, and Ba. List the samples in order of the amount (moles) from smallest to largest. O Ba < Cr < Li < Ca O Ba < Cr < Ca < Li O Ba < Ca < Cr< Li O Li < Ca < Cr < Ba Time+Management.pdf Open file Time+Management.pdf Open file Time Management a.pdf Open file 09.07.21 - Time Ma.ppt Open file ... e Type here to searcharrow_forward(3d53c Maps T T TI 14 ancing Equations Challeng ical formula. ient. h chemical formula. CO2 How many atoms of each element are in the formula shown? 0= 2Na,SO, ach element a shown? How many atoms cach are in the formula Na =arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning