
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
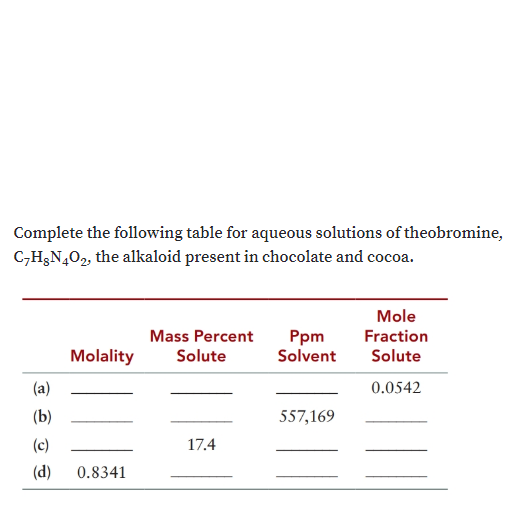
Transcribed Image Text:Complete the following table for aqueous solutions of theobromine,
C-H3N402, the alkaloid present in chocolate and cocoa.
Mole
Mass Percent
Fraction
Ppm
Solvent
Molality
Solute
Solute
(a)
0.0542
(b)
557,169
(c)
17.4
(d)
0.8341
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 6 images

Knowledge Booster
Similar questions
- A particular solute is dissolved in a solvent resulting in a solution that is 54.0 % solute by mass. If the molar mass of the solute is 178.10 g/mol, what is the molality of the solution? (Assume the solute is a nonelectrolyte).arrow_forward3.) What is the molarity of a 7.236 molal solution of potassium carbonate with a density of 1.540 g/mL?arrow_forwardHow many moles of solute are present in 75.0 grams of an aqueous solution that is 1.5% sucrose (C12H22O11) by mass?arrow_forward
- What is the molality (m) of Br - ions in 5.56 % by mass MgBr2(aq)? The molar mass of magnesium bromide is 184.113 g/mol.arrow_forwardWhat are the strongest solute-solvent interactions present in an aqueous solution of magnesium chloride?arrow_forwardWhat is the expected osmotic pressure of a 0.05 M solution of FeCl₃ in water at 20 deg C?arrow_forward
- 49. This is the principle that best describes the colligative property represented by the illustration. a. The addition of a volatile solute lowers the vapor pressure of a solution. O b. The addition of a volatile solvent lowers the vapor pressure of the solution. O c. The addition of a non-volatile solute lowers the vapor pressure of a given solution. d. The addition of a non-volatile solvent lowers the vapor pressure of a given solution 50. This is to establish an equilibrium within the solution of salt water." a. Add more salt to the solution. b. Heat the solution to boiling. O c. Close the container. d. Add more water to the solutionarrow_forwardWhat is the molality of nitric acid in a concentrated solution of nitric acid (68.0% HNO3 by mass)?(a) Outline the steps necessary to answer the question.(b) Answer the question.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY