
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Solve both subparts plz, should correct
Transcribed Image Text:freq
20
M
req
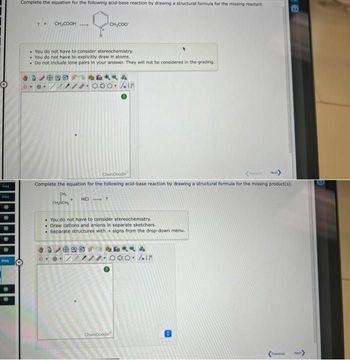
Complete the equation for the following acid-base reaction by drawing a structural formula for the missing reactant.
0
? +
CH₂COOH
• You do not have to consider stereochemistry.
You do not have to explicitly draw H atoms.
Do not include lone pairs in your answer. They will not be considered in the grading.
98
CH₂
CH₂CH₂
CH₂COO
ChemDoodle
Next>
Complete the equation for the following acid-base reaction by drawing a structural formula for the missing product(s).
HCI ?
0 ®.
4
Jall
You do not have to consider stereochemistry.
Draw cations and anions in separate sketchers.
. Separate structures with + signs from the drop-down menu.
ChemDoodle
日文文的
30-/IF
Previous
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- CUTICLE OIL O Microsoft W final E.A 1. G fahrenheit x 9 Learning M X O Schoology x O ch 9 key A mukilteo.schoology.com/common-assessment-delivery/start/4592487484?action on.. Bb Molar Mie O Periodic x 3-6, 6-5, & 6-6 Test 6 of 10 © 29 POSSIBLE POINTS: 2 What is the mass of a 5.521 mole sample of MgCl2? O 17.26 g O 100.7 g O 525.7 g O 256.1 g 4. 6 8 9 10 Lenovo DI 23 $ 7 4 t r e ーの づarrow_forwardwhat Conne X M Inbox S Therm G to cale S The at X X ducation.comext/map/index.html?_con3con&external_browser3D0&launchUrl%=https smb Tube 9 Maps E essay 1 - Google D.. view i Saved Check my work Enter your answer in the provided box. Recall Planck's constant equals 6.63 x 1034 J's and the speed of light is 3.00 x 10° m/s. Calculate the wavelength (in nm) of a photon emitted by a hydrogen atom when its electron drops from the n =5 state to the = 4 state. nm or anything DOLL 立arrow_forwardCaps Tab Wall wen M Inbox (1.600)-ftantiudeledux Mail-Francesca A Tantillo-Out xEXP #12: Geometry-CHM150-2 x Aktiv Chemistry ← → C app.101edu.co 90°F Mostly sunny 1 D Q Z Uranium hexafluoride, UF, is an important compound used in the enrichment of uranium by gaseous diffusion. A A Graham's Law states that rate of effusion A/rate of effusion of B = (square root of Mass of B)/(square root of Mass of A). Calculate how fast 235 UF gas diffuses compared to 230UF. gas. Assume the temperature remains constant. 2 W S 3 E D # C $ 4 R OL F C ▷ll s FS % 5 T V C Question 19.c of 23 G P A 6 Y B H & 7 PrtScn N J 8 N Home 1 M ( 9 K End o O < 1 4 7 +/- PgUp 0 L times faster than 238UF 2 5 8 12 4 P 3 6 9 0 □ A PED PgOn 1924 G + 0 Update Submit C x 100 BE 5:22 PM 7/6/2022 Del Backspace 과arrow_forward
- Express the following points given in Cartesian coordinates in terms of spherical coordinates. (x, y, z): (1, 0, 0); (0, 1, 0); (0,0,1); (0,0,-1)arrow_forwardA www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IJgXZp57itWHhRgilODc5Mqv... G Apps CSU Study/Teach Abro... GACE Testing O Degree Related E E-Tandem E Grade Calculator 国Re Korean O STATES OF MATTER Identifying the intermolecular forces between atoms, ions and... Kyli. What kind of intermolecular forces act between a bromine (Br,) molecule and a tetrachloroethylene (C,Cl4) molecule? Note: If there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.arrow_forward3:04 A) H₂ Which of the following molecules would exhibit the most ideal behavior? B) 0₂ C) N₂ D) He Question 19 of 21 E) Ne Submit Tap here or pull up for additional resourcesarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY