
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Complete the electron‑pushing mechanism for the given base‑promoted hydrolysis of an ester. Add atoms, bonds, nonbonding electron pairs (lone pairs), charges, and curved arrows where indicated.
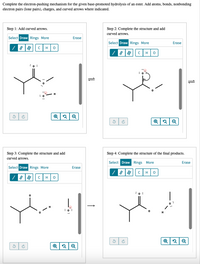
Transcribed Image Text:Complete the electron-pushing mechanism for the given base-promoted hydrolysis of an ester. Add atoms, bonds, nonbonding
electron pairs (lone pairs), charges, and curved arrows where indicated.
Step 1: Add curved arrows.
Step 2: Complete the structure and add
curved arrows.
Select Draw Rings More
Erase
Select Draw Rings More
Erase
C
H
H
:0 :
H
Step 3: Complete the structure and add
curved arrows.
Step 4: Complete the structure of the final products.
Select Draw Rings More
Erase
Select Draw Rings More
Erase
H
C
H
:0 :
|
:0 :
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name ketones and aldehydes, and draw the structures from their names.Identify their hydrates, acetals, imines, and other derivativesarrow_forwardWhen a carboxylic ester reacts with water to produce an alcohol and a carboxylate (or carboxylate salt) as the products, the reaction is called _, and requires Occur. acid-catalyzed ester hydrolysis; strong acid esterification; strong base esterification; strong acid saponification; strong acid saponification; strong basearrow_forwardWrite the reaction of an Alkyl halide ( any) with Ammonia.arrow_forward
- First, draw your chosen compounds including any non-bonding electrons and non-zero formal charges Circle and name any pH-sensitive functional groups. Choose an organic solvent that will dissolve both or your compounds and an aqueous solution (i.e. conc. aqueous NaOH, 5% aq. NaHCO3, or 3M HCl) that would successfully separate the two compounds selected.arrow_forwardFollowing ester (methyl benzoate) was hydrolyzed in presence of an acid catalyst. This reaction produces --- and ---. Question 28 options: benzoic acid, ethanol benzoic acid, water acetic acid, benzene benzoic acid, methanolarrow_forwardAlkyne can play as acid in presence of strong base. After deprotonation, what is the hybridization of the carbon who forms anion?arrow_forward
- Need help with 1-4 please. Will submit another time as well. Pre-laboratory questionsarrow_forwardHydroxide acts as a nucleophile in protein degradation by hydrolysis of the peptide bond in water. Draw out an arrow pushing mechanism of the hydrolysis of a peptide bond. Identify the nucleophile and the electrophile in this reaction.arrow_forwardComplete the electron‑pushing mechanism for the base‑promoted hydrolysis of an ester shown in the panels. Add atoms, bonds, nonbonding electron pairs (lone pairs), charges, and curved arrows.arrow_forward
- Can you help me with part A and part B, please?arrow_forwardIn a reaction where a carboxylic ester reacts with water to produce an alcohol and a carboxylate (or carboxylate salt) as the products, the reaction is called ___ , and requires ___ to occur. esterification; strong base saponification; strong base saponification; strong acid acid-catalyzed ester hydrolysis; strong acid esterification; strong acidarrow_forwardOa) An amine can be formed when 2-iodopentane is heated with ammonia in a sealed tube. This is an example of a nucleophilic substitution reaction. i) Provide a full equation (using condensed formula) for this reaction. You should name the reactants and the overall products as part of your response. ii) Name the organic product formed from this reaction and draw it's displayed structure. iii) Provide a 'curly arrow' mechanism (in displayed formula) with an accompanying explanation for this process. iv) Describe a characteristic test (with the result that would be observed) to show the presence of the amine produced upon completion of the nucleophilic substitution reaction. b) The amine produced in the above reaction forms a mixture of two optical isomers. i) Draw the 3D structures of the two isomers of the amine product that you named and produced a drawing of in part a ii). ii) With reference to a different named example, explain the term 'optical isomer'. You should draw the 3D…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY