
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:History
Aktiv Che X
Bookmarks Tools Window
3
https://app.101edu.co
Help
o Mail - Thom P Parchment
22
80
F3
$
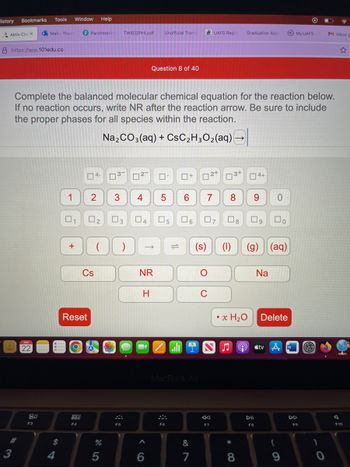
Complete the balanced molecular chemical equation for the reaction below.
If no reaction occurs, write NR after the reaction arrow. Be sure to include
the proper phases for all species within the reaction.
Na₂CO3(aq) + CsC₂H3O₂(aq) -
1
+
04-
F4
Reset
2
0₂
Cs
(
%
5
TW6D2PHI.pdf Unofficial Transi
3 4
3
F5
☐
0 4
) ->>
Question 8 of 40
NR
H
M
A
6
0
5
05 0 6
=
F6
☐+
02+
6 7 8 9
MacBook Air
87
&
7
UAFS Regist Graduation Appl
07
C
F7
3+ 4+
C 8
(s) (1) (g) (aq)
• x H₂O
*
8
9
9
FB
Na
0
☐
stv
0
Delete
O
My. UAFS-S M Inbox
F9
Ⓡ
F10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student collecting CaCO3 produced by the reaction of Na₂CO₂ (aq) and CaCl₂ (aq) obtains a percent yield of 81%. Choose all of the following observations that could explain the low yield. The precipitate was not washed prior to drying. The filter paper was not wetted with water prior to filtering the precipitate. The student did not completely dry the precipitate before weighing it. A rubber policeman was not used to scrape precipitate from the beaker. The combined reactants were not stirred before filtering the precipitate. None of the above.arrow_forwardWhy was this wrong and how do I get the right answer?arrow_forwardChilean niter deposits are mostly sodium nitrate, but they also contain 0.3%iodine in the form of calcium iodate, Ca(IO3)2. After the sodium nitrate inthe niter is dissolved and recrystallized, the remaining solution contains 9 g/Lsodium iodate, NaIO3(aq). The NaIO3 is converted to iodine when it reactswith sulfur dioxide and water.2NaIO3 + 5SO2 + 4H2O → Na2SO4 + 4H2SO4 + I2a. How many liters of sodium iodate solution that contains 9 g of NaIO3 perliter would be necessary to form 127.23 kg of iodine, I2?b. What mass, in megagrams, of Chilean niter that is 0.3% I would benecessary to form the volume of sodium iodate solution you calculated inpart (a)arrow_forward
- Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs, write NR after the reaction arrow. Be sure to include the proper phases for all species within the reaction. CsCl(aq) + K₂PO₂(aq)arrow_forward[References] Use the References to access important values if needed for this question. When the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: Fe (s) + HCI (aq) →→→→ FeCl₂ (aq) + H₂ (g) 0arrow_forwardClassify each chemical reaction: KOH(aq) + HBrO (aq) reaction K BrO (aq) + H₂O (1) Na Cl (aq) + AgNO3(aq) → NaNO3(aq) + Ag Cl (s) 16K (s) + S₂ (s)→ 8K₂S (s) 2CH₂CH₂CO₂H (1) + 70₂(g) → 6CO₂(g) + 6H₂O(g) ✓ 6 ✓ 7 type of reaction (check all that apply) combination single replacement double replacement decomposition combination single replacement double replacement decomposition combination single replacement double replacement decomposition combination single replacement double replacement decomposition ✓8 X precipitation combustion acid-base precipitation combustion acid-base precipitation combustion acid-base precipitation combustion acid-base 9 10arrow_forward
- Can you help me with this question, please? Which one is the answer right?arrow_forwardSodium hydrogen carbonate (NaHCO3), also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid (HCI), which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO3 neutralizes excess HC1 through this reaction: HCl(aq) + NaHCO3(aq) → NaCl(aq) + H₂O(1) + CO₂(g) The CO2 gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a man suffering from indigestion can be considered to be 100. mL of a 0.029 M HCl solution. What mass of NaHCO3 would he need to ingest to neutralize this much HCl ? Be sure your answer has the correct number of significant digits. X Śarrow_forwardMagic Acid® is the commercial name for a 1:1 mixture of fluorosulfonic acid, HSO3F, and antimony pentafluoride, SbF5, that generates H2SO3F+ cations – this last species qualifies as a superacid. Can Magic Acid® be diluted in water and retain its superacid property? Justify your answer and include at least one chemical equation.arrow_forward
- Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs, write NR after the reaction arrow. Be sure to include the proper phases for all species within the reaction. CaBr2(aq)+AgNO3(aq) --->arrow_forwardBalance the following equation. What is the sum of the coefficients in the balanced equation? KCIO;(s) + P(s) - P,O10(s)+ KCI(s)arrow_forwardPredict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.) Note: you are writing the molecular, and not the net ionic equation. [] → ɖ(C103) (aq) + H₂O(1) 0-0 Śarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY