
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Question 1 of 6
Submit
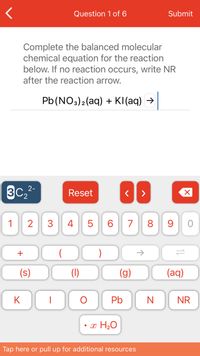
Complete the balanced molecular
chemical equation for the reaction
below. If no reaction occurs, write NR
after the reaction arrow.
Pb(NO3)2(aq) + KI(aq) →
3C,2-
Reset
1
2
3
4 5
6.
7
8 9
+
(s)
(1)
(g)
(aq)
K
Pb
NR
• x H20
Tap here or pull up for additional resources
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- what's the net ionic equation of Ag + Pb(NO3)2 =arrow_forwardComplete and balance the following acid-base and acid-carbonate reactions. (Use the lowest possible whole number coefficients.) (a) HCl + Ba(OH)2 → (b) HCl + Al2(CO3)3 → (c) H3PO4 + LiHCO3 → (d) Al(OH)3 + H2SO4 →arrow_forwardComplete the balanced molecular chemical equation. If not reaction occurs, write NR Fe(NO3)(aq) + NaOH(aq)--arrow_forward
- Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs, write NR after the reaction arrow. CsCl (aq) + K₃PO₄(aq)→arrow_forwardPredict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.) Note: you are writing the molecular, and not the net ionic equation. → NaF (aq) + H₂O(1) X O- Śarrow_forwardWrite balanced complete ionic equation for the following reaction. 2K3PO4(aq)+3CaCl2(aq)→Ca3(PO4)2(s)+6KCl(aq)arrow_forward
- Write a balanced chemical equation for this combination reaction, having correctly identified the product as Ca(OH)2 (aq)arrow_forwardcomplete the balanced molecular chemical equation for the reaction below. if no reaction occurs, write NR after the reaction arrow KOH(aq)+AlCl3(aq)=arrow_forwardBalance the following skeletal equation: Ba(NO 3) 2( aq) + K 2SO 4( aq) → BaSO 4( s) + KNO 3( aq)arrow_forward
- Predict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.) Note: you are writing the molecular, and not the net ionic equation. [] → KC1O₂(aq) + H₂O(1) X ロ→ロ 3arrow_forwardComplete the balanced molecular chemical equation for the reaction below. If no reaction occurs, write NR after the reaction arrow. BaS (aq) + Sn(NO3)4 (aq)=?arrow_forwardComplete the balanced molecular chemical equation for the reaction below. If no reaction occurs, write NR after the reaction arrow. Pb (NO3)2 (aq) + KI(aq) →arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY