
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Transcribed Image Text:Question 14 of 15
Submit
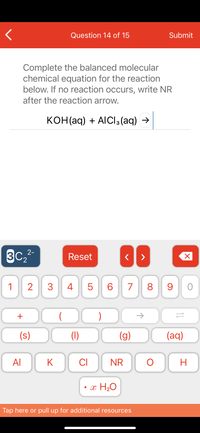
Complete the balanced molecular
chemical equation for the reaction
below. If no reaction occurs, write NR
after the reaction arrow.
конНaq) + AlCls (aq) >
3C2
2-
Reset
1
2
3.
4
6.
7
8.
9
+
)
->
(s)
(1)
(g)
(aq)
Al
K
CI
NR
H
• x H20
Tap here or pull up for additional resources
LO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs, write NR after the reaction arrow. Zn(ClO4)2(aq) + Na2S(aq) ->arrow_forwardComplete the ionic equation and the net ionic equation for this reaction. Include all the physical states, and circle the spectator ions in the complete ionic equations.arrow_forwardLabel the following equation as A Precipitation reaction, An acid base neutralization reaction or a redox reaction. 2 Al(OH)3 (aq) + 3 H2SO4 (aq) → Al2(SO4)3 (aq) + 6 H2O (l) Group of answer choices A Precipitation reaction A redox reaction An acid base neutralization reactionarrow_forward
- Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs, write NR after the reaction arrow. Cr2(SO4)3 (aq) + (NH4)2CO3 (aq) ->arrow_forwardComplete the balanced molecular chemical equation for the reaction below. If no reaction occurs, write NR after the reaction arrow. Bas(aq) + Sn(NO:)«(aq) →arrow_forwardHow many moles of acid and base are needed to produce 2.50 moles in the following equation. 3 Mg(OH)2 (s) + 2H3PO4(aq)=>Mg3(PO4)2 (s) + 6 H2O (l)arrow_forward
- Complete the balanced molecular chemical equation. If not reaction occurs, write NR Fe(NO3)(aq) + NaOH(aq)--arrow_forwardAcetylsalicylic acid (C₂H8O4) is a monoprotic acid commonly known as aspirin. A typical aspirin tablet, however, contains only a small amount of the acid. In an experiment to determine its composition, an aspirin tablet was crushed and dissolved in water. It took 5.10 mL of 0.1466 M NaOH to neutralize the solution. Calculate the number of grains of aspirin in the tablet. (One grain = 0.0648 g and the molar mass of aspirin = 180.15.) mol Round your answer to 3 significant digits. grains ☐ ☐x10arrow_forward) Write the following equation as a complete ionic equation and a net ionic equation: Note: The liquid & solid do not break into ions. Br2(ℓ) + 2NaI(aq) ---> I2(s) + 2NaBr(aq)arrow_forward
- Using your knowledge of and bases, write balanced equations for the following acid-base reactions and indicate which reactant is the acid and which is the base. Write the molecular, ionic, and net ionic equations. HNO3 (aq) + Ca(OH)2 (aq) → H2SO4 (aq) + KOH (aq) → CH3COOH (aq) + NaOH (aq) →arrow_forwardComplete the balanced molecular chemical equation for the reaction below. If no reaction occurs, write NR after the reaction arrow. CaBr2(aq) + AgNO3(aq) ->arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY