
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
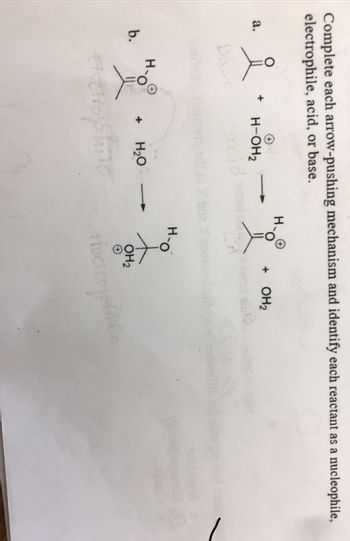
Transcribed Image Text:Complete each arrow-pushing mechanism and identify each reactant as a nucleophile,
electrophile, acid, or base.
a.
b.
요
Base
H-OH₂
+ H₂O
extrophine
H-O
+ OH₂
OH₂
он
rivcicomplice
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- COOH O 3-carboxytoluene 3-methylcyclohexanecarboxylic acid O m-methylbenzoic acid 3-toluenebenzoic acid f5 f6 %23 17 & 7 Q W R T Y # Marrow_forwardWrite out the expected splitting pattern for hydrogens Ha, Hp and Hc 3Jab = 10 Hz На Hb 3Jpc = 7 Hz CI Hc Hc Ha:Hb:Hc: doublet, quartet, doublet Ha:Hb:Hc: quartet, quartet, triplet Ha:Hb:Hc: doublet, doublet of triplets, doublet Ha:Hb:Hc: doublet, triplet of doublets, doubletarrow_forwardHh.118.arrow_forward
- The order of increasing acidity is Phenol < meta-cyanophenol < ortho-cyanophenol 1.how about para-cyanophenol??does it has higher acidicity than both ortho-cyanophenol and meta cyanophenol or lower?? 2.why does ortho-cyanophenol have higher acidicity than meta cyanophenol?? 3. why doesmeta cyanophenol have higher acidicity than Phenolarrow_forwardBelow is a structure of Levonorgestrel (Plan B®), used in prevention of pregancy. но HH H. Levonorgestrel Plan B® Below is one of the reactions used in the synthesis. What type of reaction is it? он :CEC-H Lc=c-H THF as solvent Levonorgestrel H,CO H,CO O A grignard reaction. Nucleophilic acyl substitution. O Nucleophilic addition. O An SN2 reaction. O Oarrow_forwardDraw the organic product of the Bronsted acid-base reaction. Include all lone pairs and charges as appropriate. Ignore any counterions. H3O+ Select to Draw H Z: Harrow_forward
- Which N atom in each compound is more basic? What product is formed when each compound is treated with HCl?arrow_forwardDraw the products when each pair of compounds is treated with CH3CH2O−, CH3CH2OH in a Robinson annulation reaction.arrow_forward12) Which compound is stronger base? a) b) D A d) all the samearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY