
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
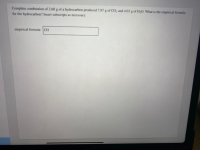
Transcribed Image Text:Complete combustion of 2.60 g of a hydrocarbon produced 7.87 g of CO, and 4.03 g of H;O. What is the empirical formula
for the hydrocarbon? Insert subscripts as necessary.
empirical formula: CH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A compound is known to consist solely of carbon, hydrogen, and oxygen. Through elemental analysis, it was determined that the compound is composed of 26.68% carbon and 2.24% hydrogen. a) What is the empirical formula of this compound? C: Empirical Formula: CoHoO0 H: Mol. Weight: b) What is the molecular weight of the empirical formula? C: c) If the molar mass of this compound is 90.04 g/mol, what is its molecular formula? 0: H: Molecular Formula: C.H.O0 O g/molarrow_forwardComplete combustion of 3.10 g of a hydrocarbon produced 9.55 g of CO, and 4.40 g of H,O. What is the empirical formula for the hydrocarbon? Insert subscripts as necessary. empirical formula: CHarrow_forwardAn Unknown compound contains only carbon, hydrogen, nitrogen, and oxygen (C, H, N, & O). Complete combustion of 3.54 g of pure compound in pure oxygen produced 8.49 g CO2, 2.14 g H2O, and 0.300 g N2. What is the empirical formula for benzocaine?arrow_forward
- Complete combustion of 3.40 g3.40 g of a hydrocarbon produced 10.9 g10.9 g of CO2CO2 and 3.89 g3.89 g of H2O.H2O. What is the empirical formula for the hydrocarbon? Insert subscripts as necessary. empirical formula:arrow_forwardWithout using a calculator, which has the greater mass: One mole of carbon atoms or one mole of chlorine atoms? Explain your reasoning. What is the benefit of using moles when working with atoms and molecules in a lab setting? How does this relate to the concept of molarity?arrow_forwardWhen 5.00 g of methane, CH4 reacts with an excess of oxygen in a combustion reaction, water and carbon dioxide are produced. What quantity of H2O is produced?arrow_forward
- When 5.555 grams of a hydrocarbon, CxHy, were burned in a combustion analysis apparatus, 17.43 grams of CO2 and 7.138 grams of H2O were produced.In a separate experiment, the molar mass of the compound was found to be 28.05 g/mol. Determine the empirical formula and the molecular formula of the hydrocarbon. Empirical formula= Molecular formula=arrow_forwardIn a first expirement, a compound is analyzed and found to contain 85.63% C and 14.37% hydrogen by mass. Determine the empirical formula of the compound. In a separate expirement, the molar mass of the compound from the first expirement was found to be 42.081 g/mol. What is the molecule formula for the compound?arrow_forwardPlease don't provide handwritten solution......arrow_forward
- Mesitylene is a liquid hydrocarbon. Burning 0.795 g of the compound in oxygen gives 2.62 g of CO2 and 0.716 g of H2O. What is the empirical formula of mesitylene?arrow_forwardWrite a balanced equation for the Alka Seltzer reaction where aqueous citric acid, H3C6H5O7, and aqueous sodium bicarbonate, NaHCO3, react and form aqueous sodium citrate, liquid water, and carbon dioxide gas.arrow_forwardPure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. When 4.78 g of magnesium ribbon burns with 7.61 g of oxygen, a bright, white light and a white, powdery product are formed. Enter the balanced chemical equation for this reaction. Be sure to include all physical states. equation: What is the limiting reactant? охудen O magnesium The reaction goes to completion, but in the process of recovering the product, some of it was lost. The the percent yield for the reaction is 82.8%. How many grams of product are recovered? mass of product recovered: How many grams of the excess reactant remain? Assume the reaction goes to completion. mass of excess reactant:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY