
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
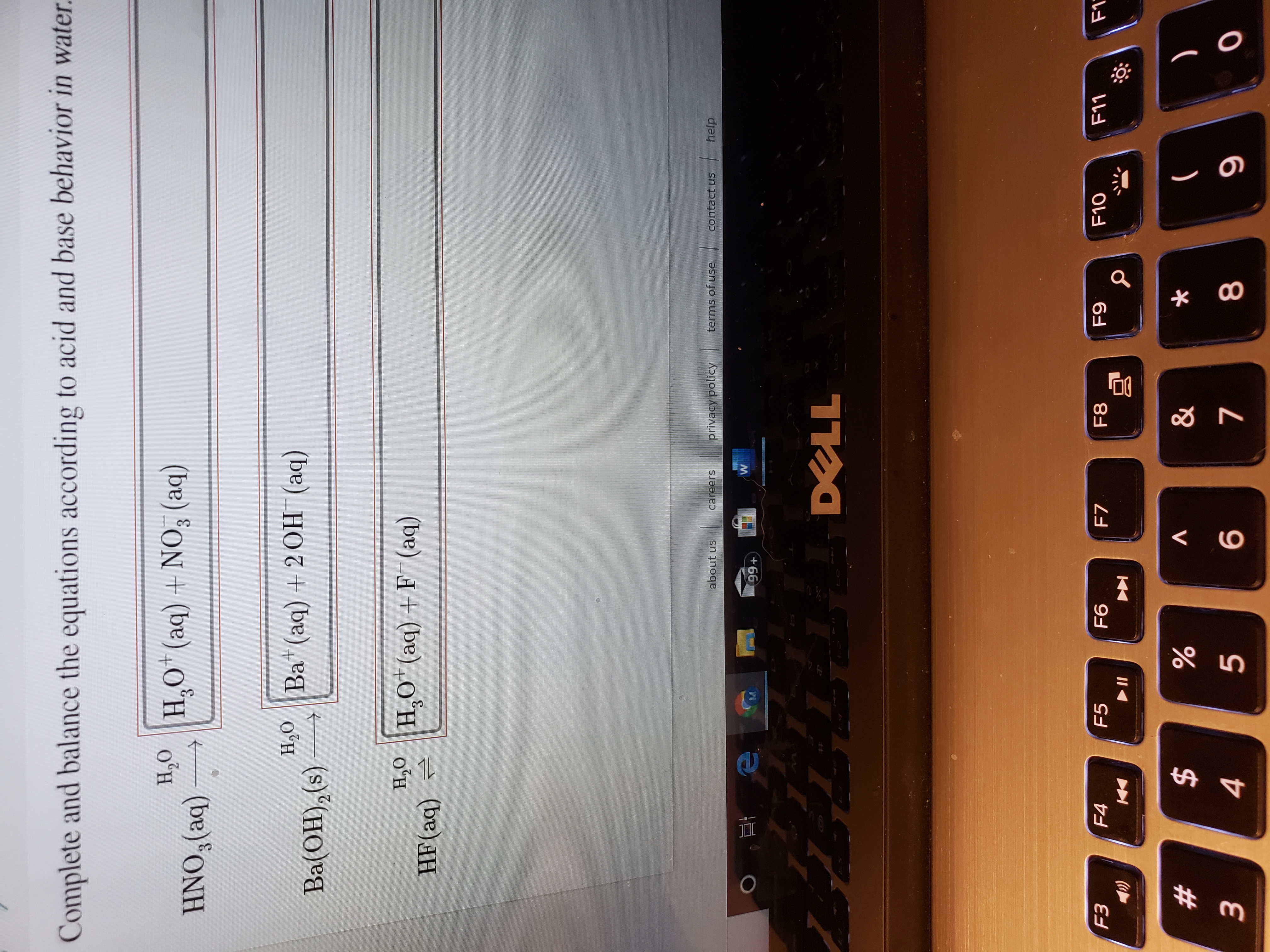
Transcribed Image Text:Complete and balance the equations according to acid and base behavior in water.
H,O
HNO,(aq)
H,O* (aq) + NO, (aq)
3.
H,O Ba+(aq) +2 OH (aq)
Ba(OH),(s)
S.
H,0 |H,o*(aq) +F (aq)
HF(aq) =
help
terms of use
contact us
privacy policy
about us
careers
99+
DELL
F3
F11
F1
F10
F9
F5
F6
F8
F4
F7
%24
&
%23
8
3
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Use the References to access important values if needed for this question. Classify each of the following as a strong acid or a weak acid. Indicate how each should be written in aqueous solution. For example, should chlorous acid be represented as HClO2 or as H++ ClO2? phosphoric acid In solution, this acid should be written as hydrochloric acid In solution, this acid should be written as acetic acid- In solution, this acid should be written asarrow_forwardConsider the following chemical equilibrium: HCOOH(aq) + H_2O(aq) \rightleftharpoons HCOO^-(aq) + H_3O^+(aq). Which of the following graphs represents the perturbation on the system and the change of pH when water is evaporated from the container?arrow_forward1.00 mL of 0.345 M HCI -Solution A. To this solution you add 123.00 mL of H2O -Solution B. i) Solution A, determine the hydronium (H^ + ) and hydroxide (OH^-) concentrations And the pH and pOH ii) Solution B determine the hydronium (H ^+ ) and hydroxide (OH) concentrations and the pH and pOHarrow_forward
- Use the References to access important values if needed for this question. The pH of an aqueous solution of 0.580 M acetylsalicylic acid (aspirin), (Ka (HC9H7O4) = 3.00 x 10^-4) isarrow_forwardCalculate the number of H+(aq)H+(aq) ions in 1.2 mLmL of pure water at 25 ∘C∘C.arrow_forwardStrong acids and strong bases ionize 100% in aqueous solution. HCl is a strong acid. In solution we write it as H"(aq) + Cl (aq). - HF is a weak acid. In solution we write it as HF(aq). - KOH is a strong base. In solution we write it as K*(aq) + OH (aq). - NH3 is a weak base. In solution we write it as NH3(aq). Exception: Since Ca(OH), is only slightly soluble we write it as Ca(OH)2(s). Below is a list of the 6 strong acids and 6 strong bases you should know. All other acids and bases are considered weak. Strong Bases LIOH, NaOH, КОН Strong Acids HCІ, HBr, HI HNO3 Ca(OH)2 (slightly soluble) HCIO4 Ba(OH)2 H2SO4 Sr(ОН)2arrow_forward
- Strong acids and strong bases ionize 100% in aqueous solution.- HCl is a strong acid. In solution we write it as H+(aq) + Cl -(aq).- HF is a weak acid. In solution we write it as HF(aq).- KOH is a strong base. In solution we write it as K+(aq) + OH -(aq).- NH3 is a weak base. In solution we write it as NH3(aq).Exception: Since Ca(OH)2 is only slightly soluble we write it as Ca(OH)2(s).Below is a list of the 6 strong acids and 6 strong bases you should know. All other acids and bases are considered weak. Strong Acids Strong Bases HCl, HBr, HI LiOH, NaOH, KOH HNO3 Ca(OH)2 (slightly soluble) HClO4 Ba(OH)2 H2SO4 Sr(OH)2 Question 1)What is the hydronium ion concentration in an aqueous hydrobromic acid solution that has a pOH of 10.200?[H3O+] = Marrow_forwardBarbituric acid, HC4H3N2O3, is used ito prepare various barbiturate drigs (used as sedaives). Without solving the quadratic equation, calculate the concentration of hydrogen ion in a (2.30x10^-1) M solution of the acid. The value of Ka is 9.8x10-5. Express your answer to three significant figures. ------------------------------------------------------------------------------------------------------------------------ What is the concentration of hydroxide ion in a (7.20x10^-2) M aqueous solution of methylamine, CH3NH2? The Kb for methylamine is 4.4x10-4. You need to solve the quadradic equation for this problem. Express your answer to three significant figures. ------------------------------------------------------------------------------------------------------------------------ A solution of lactic acid, HC3H5O3, on a laboratory shelf was of undetermined concentration. If the pH of the solution was found to be (2.51x10^0), what was the concentration of the lactic acid? The Ka of…arrow_forwardPlease help I'm not understanding!arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY