Question
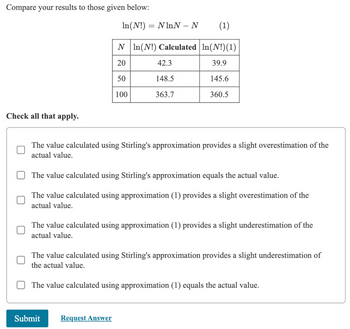
Transcribed Image Text:Compare your results to those given below:
ln(N!) = NhnN-N
(1)
N In(N!) Calculated | In(N!)(1)
20
42.3
39.9
50
148.5
145.6
100
363.7
360.5
Check all that apply.
The value calculated using Stirling's approximation provides a slight overestimation of the
actual value.
The value calculated using Stirling's approximation equals the actual value.
The value calculated using approximation (1) provides a slight overestimation of the
actual value.
The value calculated using approximation (1) provides a slight underestimation of the
actual value.
The value calculated using Stirling's approximation provides a slight underestimation of
the actual value.
The value calculated using approximation (1) equals the actual value.
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- The image shows the example of finding the number of vacancies in 1 cubic meter of copper (Cu) at 1000 degrees celcius (1273 k) considering the image data. Replicating the problem in the image, calculate the number of vacancies but at room temperature.Explain why there is such a difference in the number of vacancies at both temperatures.arrow_forwardPlease solve the problem in the picture, thanks in advance :)arrow_forwardConsider a system with 1000 particles that can only have two energies, ɛ, and with ɛ, > E,. The difference between these two values is Aɛ = ɛ, -& . Assume that gi = g2 = 1. Using the %3D %3D equation for the Boltzmann distribution graph the number of particles, ni and m, in states & n2, E and E, as a function of temperature for a Aɛ = 1×10-2' J and for a temperature range from 2 to 300 K. (Note: kg = 1.380x10-23 J K-!. %3D %3D (s,-s,) gLe Aɛ/ n2 or = e n,arrow_forward
arrow_back_ios
arrow_forward_ios