
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
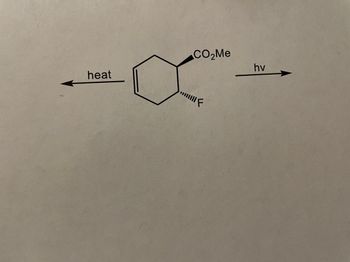
Transcribed Image Text:CO₂Me
hv
heat
"F
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- A 200.0 g of tin released 394 calories (cal.) of heat when cooled from 100.0°C to 24.5°C. What is the specific heat of the metal? A) 0.0394 cal/(g x °C) B) 0.0261 cal/(g x °C) C) 19.2 cal/(g x °C) D) 5.220 cal/(g x °C) E) 0.161 cal/(g x °C)arrow_forwardIdentify the point(s) on the following diagram where the addition of heat will cause the temperature of the sample to increase. Temperature B C Point(s): E LL F Heat Energy H Enter your answer in alphabetical order, e.g., DFJ not FDJ.arrow_forwardA 275 g sample of metal requires 10750 J of heat to change the temperature 306° C. What is the specific heat? Record answer with correct sig figs and no unit.arrow_forward
- how whould i calculate the specific heat of the mental in (J/gC)arrow_forwardneed help with this!arrow_forwardAccording to the following thermochemical equation, how much heat is produced when 3.85 g of CHL is burned in excess O2. . Fall 2 CH (8) + 2 O2 (g) CO2e+2 H,O AH +2 H2O (g) AH = 802 kJ 192KJ b. 385 kJ C. 802 kJ d. 96KJ e. Not listed 5. 0OMHCL what volume of the 12 M HCl do you need? You have a solution of 12 M HCI. f you want to dilute this to a 100. mL soluarrow_forward
- Can some show me how to calculate the specific heat with the given information?arrow_forwardA chemical reaction takes place inside a flask submerged in a water bathThe water bath contains 2.80 kg of water at 21.5 degrees * C During the reaction 110. of heat flows out of the flask and into the bath Calculate the new temperature of the water bathYou can assume the specific heat capacity of water under these condition 4.18 3* g^ -1 K^ -1 Be sure your answer has the correct number of significant digits.arrow_forwardHow much heat energy is required to raise the temperature of 0.361 kg of copper from 23.0 °C to 60.0 °C? The specific heat of copper is 0.0920 cal/(g ·°C). Express your answer with the appropriate units. • View Available Hint(s) HÀ ? heat = Value Unitsarrow_forward
- Please atleast answer this questionarrow_forwardOA 850ngaL Sample of water was Cooleal from loo°c to 10&, How much heat was lost. collecilgnA in lla atoslan BCCC z qea okymo olmoo adi liem3 E ilgge to oisc bou to indse tol oaillbesb orT O mg 2.11 ECCGERarrow_forwardI got tangle up with this question . Can u please explain to me how to resolver . thanks !arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY