
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
1. Name the amino acids present in this peptide bond.
2. The structure shown represent a major ingredient in some commerial product. Identify the name of this major ingredient and the commercial product.
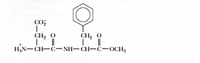
Transcribed Image Text:CO
CH2 O
|
CH2 O
||
H3N-CH-C- NH- CH-C-OCH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 12. The sugars glucose, fructose, and inositol all have the same formula i.e. C6H12O6 yet they have 3 very similar but different structures. This is an example of: a) geometric isomers b) structural isomers c) stereo isomers d) none are correctarrow_forwardselect all that applyarrow_forwardDiscuss using your own words and illustrations the organisation of a protein, from its linear amino acid sequence, to its final three–dimensional configuration, which may include one or more polypeptides. Include what types of bonds help to stabilise each structural level, from primary through quaternary structure.arrow_forward
- In the space below draw a dipeptide composed of lysine and glutamic acid bound together by peptide bondarrow_forwardwhich of the following is not correct about peptide bonding? A- It occupies a single plane B- It can associate via H-bonding C- It has a double bond character D- It exhibits geometric isomerismarrow_forwardSelect the properties that apply to the secondary structure beta-pleated sheets (select all that apply). can be Antiparallel can be Parallel are right handed Oare left handed obeys n+4 rule are formed by the pattern of hydrogen bonding the backbone makes with itself are formed by the pattern of covalent bonds the backbone makes with itself are formed by the pattern of electrostatic attractions the backbone makes with itselfarrow_forward
- Draw a peptide bond between two generic amino acids at pH 7; use R1 and R2 for the side chains. include all atoms and bonds in the two amino acide Make the structure is big enough to see cleany.arrow_forwardPlease describe what is a peptide bond? What is the significance of the amino terminus versus the carboxy terminus? At which end are amino acids added to form a polypeptide, this is the term for linked amino acids? What are the 3 chemical groups that form an amino acid? A▾ B I I @ & 7arrow_forward1. Proteins perform critical functions in all of our cells. Without proteins, life wouldn’t exist. Think of some specific proteins and describe what function they perform. 2. Explain the difference between secondary and tertiary protein structures. 3. How many water molecules are produced when a dipeptide is synthesized?arrow_forward
- Match the areas of a dipeptide with the labeling number. The N-terminus nitrogen: The C-terminus carbon: The two central (a.) Carbons: and The two side chains: and The peptide bond: between and 2 3 H (N: -C. N- 5 8. H12 CH3 13arrow_forward2. a. In the following diagram of a portion of a protein, label the types of interactions that are shown. b. What level of protein structure are these interactions producing? b. CH CH3 CH3 Polypeptide backbone CH2 H3C H3Ç 10 CH С—ОН - CH,-S-S-CH,- а. CH2 с. -CH,-CH,-CH2-CH2-NH 0-C-CH2- d.arrow_forward3. Which best describes the contribution of tertiary (3’) structure of to the native conformation of polypeptides and proteins: a) Structure that results from intrachain interactions of amino acid side chains b) Structure that results from interchain interactions of amino acid side chains c) Structure that results from base pairing d) Structure that results from the linear sequence of amino acids from beginning to end of moleculearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON