
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
what are the oxidizing agents in order starting strongest to weakest
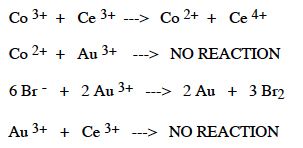
Transcribed Image Text:Co 3+ + Ce 3+ ---> Co 2+ + Ce 4+
Co 2+ + Au 3+
---> NO REACTION
6 Br + 2 Au 3+ > 2 Au + 3 B12
Au 3+ + Ce 3+
---> NO REACTION
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- Animation-Analysis of Copper-Zinc Voltaic Cell Zinc Some oxidation-reduction reactions are spontaneous, and the energy released by them can be used for electrical work. This principle is used in the working of a voltaic cell. Voltaic cells, or electrochemical cells, make use of the electrons that are transferred from the species that releases electrons and undergoes oxidation to the species that accepts electrons and undergoes reduction. If it is possible by any means to separate the oxidation reaction and the reduction reaction and then connect them externally so that the electrons flow from one compartment to the other, you can construct an electrochemical cell. Watch the video that describes the cell reaction and the cell components of a voltaic cell. 1.10 14 of 42 Na₂SO₂(aq) Review | Constants | Periodic Table Z 5042 Cu2+ $0,² In a copper-zinc voltaic cell, one half-cell consists of a Zn electrode inserted in a solution of zinc sulfate and the other half-cell consists of a Cu…arrow_forwardFor any polyatomic ion, the sum of the oxidation mumbers equals zero. Is this true or falsearrow_forwardWhen the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown? Zn²+ + H3ASO3 Zn + H3ASO4 Water appears in the balanced equation as a for neither.) How many electrons are transferred in this reaction? (reactant, product, neither) with a coefficient of (Enter 0arrow_forward
- H2. In what type of reaction is water always a product? O decomposition O oxidation O synthesis O precipitation O acid-base Please explain with details also explain wrong optionsarrow_forwardWhen the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown? A1³+ + Fe2+ AI + Fe3+ Water appears in the balanced equation as a (reactant, product, neither) with a coefficient of (Enter 0 for neither.) How many electrons are transferred in this reaction?arrow_forwardThe species in a redox reaction that becomes reduced is the catalyst product reducing agent oxidizing agentarrow_forward
- Identify the species oxidized, the species reduced, the oxidizing agent and the reducing agent in the following electron-transfer reaction.2Cl-(aq) + F2(g) Cl2(g) + 2F-(aq) species oxidized species reduced oxidizing agent reducing agent As the reaction proceeds, electrons are transferred from to .arrow_forwardWhen the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown? NO+ Cu2+ NO₂ + Cut (reactant, product, neither) with a coefficient of. (Enter 0 for Water appears in the balanced equation as a neither.) How many electrons are transferred in this reaction?arrow_forwardA solution of chromium, nitrate gives an acid reaction because the hydrated chromiumun ion tends to lose a proton from one of the water molect complexed with it. nitrate ions react with water to form the strong acid, nitric acid nitrate ions act as a strong pxidizing agent nitrate ions are acidic in water.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY