
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
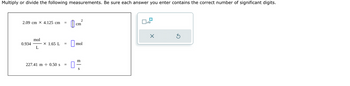
Transcribed Image Text:Multiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits.
2.09 cm X 4.125 cm =
0.934
mol
L
X 1.65 L =
227.41 m 0.50 s =
cm
2
mol
m
S
x10
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the masses and volumes indicated, calculate the density in grains per cubic centimeter. mass = 452.1 g: volume = 292 cm3 mass = 0.14 lb: Volume 125 mL mass = 1.01 kg; volume = 1000 cm3 mass = 225 mg: volume = 2.51 mLarrow_forwardFor these questions, be sure to apply the rules for significant figures. a You are conducting an experiment where you need the volume of a box; you take the length, height, and width measurements and then multiply the values together to find the volume. You report the volume of the box as 0.310 m1. If two of your measurements were 0.7120 m and 0.52145 m, what was the other measurement? b If you were to add the two measurements from the first part of the problem to a third length measurement with the reported result of 1.509 m, what was the value of the third measurement?arrow_forwardMultiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits.arrow_forward
- Multiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 0.93 cm x 3.00 cm = 257.9 g + 0.52 mL = 0 20.94 g mL 0 × 53. mL = 2 cm mL 08 0x10 Xarrow_forwardMultiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 7.81 cm X 2.025 cm = 369.17 g 68.0 mL 539.7 m 0.68 s = = 2 cm² 0 g mL m 07/23 S x10arrow_forwardMultiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 0.93 cm x 4.925 cm = 358.62 g 64.52 mL = 93. g mL cm 2 g mL × 93. mL = g || x10arrow_forward
- Multiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 102.68 mol = 0.37 L = 898.8 g 41.2 mL = 2.09476 g mL X 2.3 mL = mol L g mL g x10 X Śarrow_forwardMultiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits.arrow_forwardMultiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits.arrow_forward
- Multiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 960.89 m 0.59 s = 0.934 2.09 mol L g mL × 0.25 L = x 4.55 mL m S mol 8 x10 X Sarrow_forwardMultiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits.arrow_forwardMultiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 2.09476 cm X 3.325 cm 444.8 m 0.35 s = 20.9476 cm X 43. cm cm m 2 S cm x10 Xarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning