
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
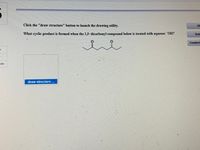
Transcribed Image Text:Click the "draw structure" button to launch the drawing utility.
Hi
What cyclic product is formed when the 1,5-dicarbonyl compound below is treated with aqueous OH?
Solu
Guided
ces
draw structure ...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- what are the structures for the follwoing c) benzoate reacts with methyl benzoate reacts with ethyl benzoate reacts with 1-propyl benzoate reacts with 2-propylarrow_forwardUse a sheet of paper to answer the following question. Take a picture of your answers and attach to this assignment. n-Pentanol (CH3CH2CH₂CH₂CH₂OH) and 2-methylbutan-2-ol (CH3CH₂C(CH3)2OH) are converted to their corresponding alkyl chorides on being reacted with hydrogen chloride. (a) Write out an equation for each reaction (b) Assign each the appropriate symbol (SN1 or SN2) (c) Write a suitable mechanism for each reactionarrow_forwardDuring the purification step of banana oil, pentane is separates from the desired product by distillation. Explain why this is possible.arrow_forward
- Click the "draw structure" button to launch the drawing utility. %3D Draw the product formed when the following compound is treated with PCC. OH CHO HO, draw structure ...arrow_forwardWhat is the product of pentene and water under acidic conditions? Group of answer choices pentanol 2-pentanone pentanoic acid pentanalarrow_forwardProvide the IUPAC name of this molecule.arrow_forward
- In the titration of 25.00 mL of a water sample, it took 19.040 mL of 4.965x 10−3 M EDTA solution to reach the endpoint. Convert the number of mL of bottled water used in each sample titration to Liters. (enter your answer with 3 significant figures)arrow_forward1. Draw structures for the following molecules. 1,5-dihydroxypentan-3-one p-nitrobenzaldehyde (3S)-4-hydroxy-3-methylbutanal 3-phenylcyclohex-2-enonearrow_forwardWhat is the major organic product in the following reaction sequence? Type its systematic IUPAC name in the box below. Br 1) NaCN 2) H3O*, heat ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY