
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
HW9 #17
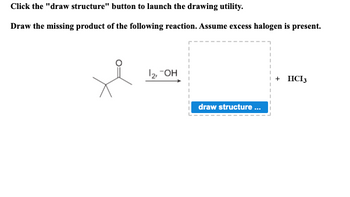
Transcribed Image Text:Click the "draw structure" button to launch the drawing utility.
Draw the missing product of the following reaction. Assume excess halogen is present.
je
1₂, -OH
draw structure...
+ HICI 3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Room temperature: 294.0 K Barometric pressure: 770.5 mmHg Vapor of water: 18.6 mmHg Volume of O2 collected: 57.90 mL Density of H2O2: 1.01 g/mL % Composition H2O2: 3.02 % Volume of H2O2 used: 5.00 mL Letter of the unknown solution of H2O2: B Volume of O2 collected for the unknown: 20.20 mL corrected barometric pressure. 752 mmHg Expert, what is a term letter?arrow_forwardFrom the reaction: B2H6 + O2 → HBO2 + H2: What volume of 60% in excess air at 1 atm and 25 deg C is needed to burn 36.1 g of B2H6?arrow_forwardA bubble of carbon dioxide gas clinging to the inside of a fish tank occupies 1.5 x 10-4 cm3. The pressure exerted on the bubble is 2.3 atm. The bubble moves to a point closer to the surface of the water where the pressure is 1.8 atm. This causes the bubble to expand to 2.2 x 10-4 cm3. This is an example of _________. Select one: a. Charles's Law b. Cole's Law c. Amonton's Law d. Avogadro's Law e. Boyle's Lawarrow_forward
- vellum.ecollege.com/course.html?courseld3D16914186&OpenVellumHMAC=f8cc2710256b118234d2cdf19573d5eb#10001 O Maps O 85951877123 Milton Ochoa - Exp.. O PRESENTACIÓN SE.. Correo: LUCIANA C. abe M Gmail SANTILLANA Item 11 Part A Automotive air bags inflate when sodium azide decomposes explosively to its constituent elements: 2 NaN3(s) , 2 Na(s) + 3 N2(g) How many grams of sodium azide are required to produce 18.0 g of nitrogen? O 41.8 g O 27.8 g O 0.964 g O 0.428 g O 62.7 g Submit Deguest Anewer P Pearson uí para buscar Escritorio 23 JUUUUUL E R G 远arrow_forwardThe name of the 9th most abundant gas in dry air at sea level is _________.arrow_forwardplease fill out column 9 given the photo providedarrow_forward
- [References] Ethene is converted to ethane by the reaction Catalyst C2H4 (9) + H2 (9) C2H6 (g) ot C2H4 flows into a catalytic reactor at 28.7 atm and 300.°C with a flow rate of 1150. L/min. Hydrogen at 28.7 atm and 300.°C flows into the reactor at a flow rate of 1450. L/min. If 14.8 kg C2 H6 is collected per minute, what is the percent yield of the reaction? pt Percent yield ptarrow_forwardHelparrow_forwardNon-STP Conditionsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY