
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
#1
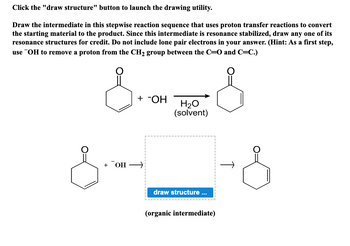
Transcribed Image Text:Click the "draw structure" button to launch the drawing utility.
Draw the intermediate in this stepwise reaction sequence that uses proton transfer reactions to convert
the starting material to the product. Since this intermediate is resonance stabilized, draw any one of its
resonance structures for credit. Do not include lone pair electrons in your answer. (Hint: As a first step,
use OH to remove a proton from the CH₂ group between the C=O and C=C.)
s-- S
+ -OH
+ OH →
H₂O
(solvent)
draw structure ...
(organic intermediate)
-&
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the major product of this acid-base reaction. Include all lone pairs. Ignore inorganic byproducts. :0: H NaNH2 Qarrow_forward3) Use the reaction shown below and the associated reaction energy diagram to answer the following questions. E A Но- + Br HO. Br- reaction coordinate a) In the space above, use curved arrows to show the mechanism of the reaction. b) Classify the reaction as either addition, elimination or substitution. c) In this reaction is hydroxide (HO-) acting as a nucleophile or as a base? c) Based on the reaction energy diagram, is the reaction endergonic or exergonic? d) Based on the reaction energy diagram, and assuming the reaction is reversible, would the equilibrium lie to the left or to the right? e) Which position on the reaction energy diagram (A, B or C) corresponds to the transition state?arrow_forwardDraw all intermediates and show all electron-pushing.arrow_forward
- Choose the one has the highest priority, and the one has the lowest. (Please show why) -CHO -COOH -COCH3 -CONH2arrow_forwardDraw the reactant of the following reaction. ? OH heatarrow_forward10:54 ← Question 21 of 32 Draw the major product of this reaction. Ignore inorganic byproducts. Submit Assume that the water side product is continuously removed to drive the reaction toward products. (CH3)2NH, TSOH Select to Draw | I Iarrow_forward
- Hello! Can someone please help me with this question?arrow_forwardAdd curved arrows to the reactants in this reaction. A double-barbed curved arrow is used to represent the movement of a pair of electrons. Draw curved arrows. Select Draw Rings More Erase | : 0 H-O: H OHarrow_forwardComplete the electron‑pushing mechanism for the given decarboxylation reaction. Add bonds, nonbonding electron pairs (lone pairs), and curved arrows where indicated.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning