
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
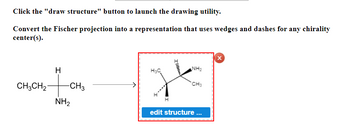
Transcribed Image Text:Click the "draw structure" button to launch the drawing utility.
Convert the Fischer projection into a representation that uses wedges and dashes for any chirality center(s).
**Diagram Explanation:**
- On the left, there is a Fischer projection of a molecular structure:
- The vertical line in the center has three groups attached to it:
- At the top, there is a hydrogen (H) atom.
- In the middle, there is a carbon (C) atom connected to a methyl group (CH3) and an ethyl group (CH3CH2).
- At the bottom, there is an amine group (NH2).
- An arrow points from the Fischer projection to the right, indicating the conversion of this structure.
- On the right, there is a 3D representation of the molecule, using wedges and dashes to depict the chirality:
- A wedge-shaped line (solid triangle) represents a bond coming out of the plane towards the viewer.
- A dashed line represents a bond going behind the plane away from the viewer.
- The molecular structure shows the same groups as in the Fischer projection, but now in a 3D perspective to convey chirality:
- The ethyl group (CH3CH2) and the amine group (NH2) are positioned with wedge and dashed lines.
- The methyl group (CH3) and hydrogen (H) are placed in the plane of the page.
- Below the 3D representation, there is a button labeled "edit structure ...", suggesting an interactive element on the educational website, allowing users to edit the molecular structure.
The diagram effectively demonstrates how to convert a 2D Fischer projection into a 3D representation to understand the chirality centers in molecules better.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4. For each of the following molecules: H₂C H₂C Me a) Label each chiral atom with its R or S configuration b) Label each molecule as chiral or achiral c) For each chiral molecule, draw its enantiomer NH₂ H RICE H CH, Br H Mearrow_forwardClick on all of the carbon chirality centers in the molecule below. (Other terms used for chirality center include chiral center, stereocenter, and stereogenic center.) If no carbon qualifies, submit your answer without selecting any. OH CH3 CH3arrow_forwardClick on all of the carbon chirality centers in the molecule below. (Other terms used for chirality center include chiral center, stereocenter, and stereogenic center.) If no carbon qualifies, submit your answer without selecting any. H. CO2H HOarrow_forward
- Draw the pyranose α-anomer chair conformation from the Fischer projection shown below.arrow_forwardThere are eight chirality centers in the following molecule. Identify each asymmetric carbon atom by clicking on the circled carbons that are chiral. HOarrow_forwardQuestion 4 of 30 O Macmillan Learning A prochirality center is a precursor to a chirality center. Identify the prochirality centers (if any) in each structure. H3C |||| ··|||| I CI CH H CH3 H == H3C. CH3 -C. CH3 Answer Bank prochirality centerarrow_forward
- Click the "draw structure" button to launch the drawing utility. Convert the Fischer projection into a center(s). CH3 CH3CH2 -Br CI representation that uses wedges and dashes for any chirality CH3 Oll H₂C Br edit structure...arrow_forwardDraw the Fischer projection for structure I. Circle each chiral group in structure II.arrow_forward3. State whether the pairs of molecules are enantiomers, diastereomers, identical, or different compounds a. b. c. H3C. H F. H H CH3 ного/. H *** Et ļ. H CH₂F CO₂H Mearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY