
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
pls help, i just need the answer for the ones blank ( the answers for the question in each picture )
![[References]
Classify the following substituents according to whether they are electron donors or electron acceptors relative to hydrogen by the resonance and the
inductive mechanisms.
1.
2.
3.
-Seh
selenyl
-SCH3
thiomethyl
-CH₂CH3
ethyl
Inductive effect [acceptor Resonance effect [donor
Inductive effect acceptor Resonance effect donor
Inductive effect acceptor Resonance effect [acceptor ✓](https://content.bartleby.com/qna-images/question/0956b951-904a-4e8f-b73a-a0aa05e6cd9c/4b1185ed-e360-44e1-98a8-53b942bc58f9/pu5pkp_thumbnail.png)
Transcribed Image Text:[References]
Classify the following substituents according to whether they are electron donors or electron acceptors relative to hydrogen by the resonance and the
inductive mechanisms.
1.
2.
3.
-Seh
selenyl
-SCH3
thiomethyl
-CH₂CH3
ethyl
Inductive effect [acceptor Resonance effect [donor
Inductive effect acceptor Resonance effect donor
Inductive effect acceptor Resonance effect [acceptor ✓
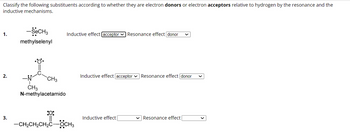
Transcribed Image Text:Classify the following substituents according to whether they are electron donors or electron acceptors relative to hydrogen by the resonance and the
inductive mechanisms.
1.
2.
3.
-SeCH3
methylselenyl
CH3
CH3
N-methylacetamido
Inductive effect [acceptor Resonance effect donor
:0:
—CH,CH,CH,COCH3
Inductive effect acceptor Resonance effect donor
Inductive effect
Resonance effect
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help me out by tick the correct option thanksarrow_forwardconfused on thisarrow_forwardFor each of the statements below, indicate whether it is true or false and explain your reasoning. (1-3 sentences) ( 1/ It takes more energy to ionize an electron from the 2s orbital than an electron from the 2p orbital in the Li2+ ion. 2/ The electron affinity of the Ne atom is larger than the electron affinity of the F atom. 3/ K* has a larger radius than Ar. 4/ For a diatomic molecule, in which the internuclear axis is the z-axis, the 3dz2 orbital cannot mix with the 2px orbital. 5/ The internuclear distance of O2 increases as it takes an additional electron to become O2.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY