
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
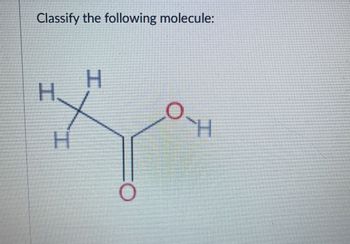
Transcribed Image Text:Classify the following molecule:
H
H
O
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Consider the molecule below. Determine the molecular geometry at each of the 2 labeled carbons. H H CC=C 1 H CI C=C CH3 F C1 tetrahedral, C2 - linear = O C1 = trigonal planar, C2- bent OC1 trigonal planar, C2 = tetrahedral O C1 = trigonal pyramidal, C2 = see-saw OC1 = bent, C2 = trigonal planararrow_forwardThe bond angles around all of the carbons in 2-methylbutane are the same because all ot the carbons in this molecule have the same "ABE" classification. Can you help me with part A and part B?arrow_forwardWhat is the H-O-C bond angle in Molecule of methanolarrow_forward
- Use the following Lewis diagram for methyl acetate to answer the questions: H :0: ++++ C -H 1 2 H H H Remember that geometry refers to the geometry defined by the atoms, not the electron pairs. The geometry about atom C₁ is The ideal value of the C-C-O angle at atom C₂ is The geometry about atom 03 is degrees.arrow_forwardUse VSEPR to predict bond angles about each highlighted atom.arrow_forwardName the skeletal formulaarrow_forward
- omplete the Lewis diagram before answering the following questions. How many sigma bonds are there in a molecule of allylcyanide? How many valence electrons are there in a molecule of allylcyanide? How many pi bonds are there in a molecule of allylcyanide?arrow_forwardA cumulene has double bonds directly connected to each other. Identify all the atoms that must lie in the same plane in the molecule shown below. Xarrow_forwardIn the molecule shown below, what is the molecular geometry for the atom marked with the arrow?arrow_forward
- Subject--chemistryarrow_forwardConsider the molecule below. Determine the molecular geometry (shape) at each of the 2 labeled carbons. H H C= 0 H CI C=C 2 CH3 F O C1 = trigonal planar, C2 = tetrahedral C1 = trigonal planar, C2= bent C1 = trigonal pyramidal, C2 = see-saw C1 = bent, C2 = trigonal planar O C1 = tetrahedral, C2 = lineararrow_forwardPlease help asaparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY